GEOLOGY AND ROCK SYSTEM
Minerals
Minerals are fundamental building blocks of the Earth’s crust and have significant importance in various fields, including geology, materials science, and economics. Here’s an in-depth look at the concept of minerals, expanding on the points mentioned:
Definition and Characteristics
- Basic Definition: Minerals are naturally occurring substances characterized by an orderly atomic arrangement and a defined chemical composition. They can be either organic or inorganic, although the vast majority are inorganic. Organic minerals, such as coal, are derived from the remains of living organisms.
- Chemical and Physical Properties: The specific chemical composition and the orderly arrangement of atoms within minerals give them a unique set of physical properties, such as color, hardness, cleavage, luster, and specific gravity. These properties are crucial for mineral identification and classification.
- Solid State: Most minerals are found in a solid state under normal Earth surface conditions. This solid state reflects a tightly bonded, orderly atomic structure. However, exceptions exist, such as mercury, which is a liquid metal at room temperature.
- Crystalline Nature: Minerals typically possess a crystalline structure, meaning their atoms are arranged in a specific, repeating pattern. This crystalline structure is responsible for the characteristic shapes and facets of mineral crystals.
Composition
- Elemental Composition: Minerals can be composed of a single element, such as gold (Au), silver (Ag), sulfur (S), and copper (Cu), or they can be compounds consisting of two or more elements. For example, quartz is composed of silicon and oxygen (SiO2), and bauxite, an ore of aluminum, contains aluminum oxide (Al2O3) along with other compounds.
- Variety of Minerals: There are over 4,000 known mineral species, each with its own chemical composition. The diversity is due to the combination of different elements in varying proportions and the conditions under which the minerals form.
Formation and Sources
- Formation from Magma: The primary source of all minerals is magma, the molten rock beneath the Earth’s surface. As magma cools, either beneath the surface or after it erupts onto the surface as lava, minerals crystallize from the melt. The cooling rate, pressure, and chemical composition of the magma determine which minerals form.
- Systematic Mineral Formation: A systematic series of minerals form through the process of fractional crystallization as molten rock cools. The sequence in which minerals crystallize from magma is predictable and is described by Bowen’s Reaction Series.
- Occurrence: Minerals are found in various geological environments throughout the Earth’s crust. They form the building blocks of rocks and are concentrated in ore deposits, which are mined for metals and other valuable resources.
Exceptions and Varieties
- Organic Minerals: While most minerals are inorganic, certain substances like coal, petroleum, and natural gas are considered organic minerals. These materials are derived from the accumulated remains of plants and animals and have undergone chemical transformations over geological time scales.
- Physical States: Although the typical image of a mineral is a solid substance, minerals can also be found in liquid (e.g., mercury) and gaseous forms (e.g., natural gas), although these are less common.
Classification of Minerals
Metallic Minerals
- Precious Minerals: These are highly valuable for their rarity and include metals such as platinum, gold, and silver. Their value makes them significant for jewelry, investment, and various industrial applications where their properties, like conductivity and corrosion resistance, are desired.
- Ferrous Minerals: Characterized by their iron content, ferrous minerals are magnetic and pivotal to the steel and manufacturing industries. Iron ore is the most prominent example, serving as the primary raw material for steel production.
- Non-Ferrous Minerals: This category includes minerals without iron content, such as copper, lead, zinc, and aluminum. These materials are essential in electrical wiring, batteries, and as components in alloys for construction and machinery.
Non-Metallic Minerals
- Examples: Minerals such as sulfur, phosphates, and nitrates fall under this category. They lack metallic content and are key in various industries, including fertilizers (phosphates and nitrates) and chemical production (sulfur).
- Cement Production: Cement is a notable example of a product made from non-metallic minerals, primarily limestone (calcium carbonate) and clay. These minerals are heated to produce clinker, which is then ground to make cement, a critical material in construction.
Physical Characteristics
Crystal Structure
- Determined by the internal arrangement of atoms or molecules in a lattice. Common crystal shapes include cubic, octahedron, and hexagonal. This structure influences many of the mineral’s physical properties.
Cleavage and Fracture
- Cleavage refers to a mineral’s tendency to break along flat, parallel surfaces, governed by its atomic structure.
- Fracture occurs when a mineral breaks in an irregular or curved surface, not along the planes of cleavage.
Lustre and Colour
- Lustre describes how light reflects from a mineral’s surface and can range from metallic and silky to glossy.
- Colour can be a distinctive feature due to the mineral’s inherent composition or caused by impurities. For example, malachite is inherently green, while fluorite’s color can vary widely due to impurities.
Streak and Transparency
- Streak is the color of a mineral in powdered form and can differ from the mineral’s surface color. This property is helpful in mineral identification.
- Transparency varies from transparent (light can pass through) to opaque (light cannot pass through). This property depends on the mineral’s internal structure.
Structure and Hardness
- Structure can describe the arrangement of a mineral’s crystals, ranging from fine to coarse-grained.
- Hardness is a measure of a mineral’s resistance to scratching, defined on a scale from 1 (talc) to 10 (diamond). This scale, known as Mohs scale of mineral hardness, is essential for identifying minerals and understanding their durability.
Specific Gravity
- Specific Gravity is a measure of density, comparing the weight of a mineral to the weight of an equal volume of water. This property can vary widely among minerals and helps in their identification.
SOME MINERALS AND THEIR USES
To provide an in-depth explanation of the minerals listed and their uses, we’ll break down each mineral into its fundamental properties, occurrence, and applications in various industries. This approach not only helps in understanding the significance of each mineral but also illustrates the diversity of their applications.
Feldspar
- Properties: Feldspar is a collective term for a group of minerals that are rich in aluminosilicates of sodium, potassium, and calcium. They are characterized by their crystalline structure and hardness of 6 on the Mohs scale.
- Occurrence: Feldspar minerals make up about 50% of the Earth’s crust, found in igneous, metamorphic, and sedimentary rocks. Their abundance makes them significant geological indicators.
- Uses: The primary use of feldspar is in the ceramic and glass industries. In ceramics, feldspar acts as a flux, lowering the vitrification temperature of the body and enhancing the strength and translucency of the product. In glassmaking, it contributes to the glass’s hardness, durability, and resistance to chemical corrosion.
Quartz
- Properties: Quartz is a hard, crystalline mineral composed of silica (SiO2). It ranks 7 on the Mohs scale, making it highly durable and resistant to weathering.
- Occurrence: It is a significant component of granite and sand, found in both igneous and metamorphic rocks. Quartz veins are also a common occurrence in various rock types.
- Uses: Beyond its widespread use in the construction industry (due to its presence in sand), quartz is crucial in the tech industry for making glass, as well as in the manufacturing of electronic devices, watches, and optical instruments. Its piezoelectric properties are utilized in radio and radar technologies for frequency control and stabilization.
Pyroxene
- Properties: Pyroxene refers to a group of inosilicate minerals with a similar crystal structure but varying chemical compositions, including calcium, magnesium, iron, silicon, and aluminum.
- Occurrence: Making up about 10% of the Earth’s crust, pyroxenes are primarily found in igneous and metamorphic rocks. They are also common in lunar rocks and meteorites.
- Uses: Although not widely used in specific industries due to their commonality and general properties, pyroxenes are studied for their role in understanding geological processes and are sometimes used as aggregate in construction.
Amphibole
- Properties: Amphibole minerals are inosilicates with a complex structure and composition, including elements like sodium, calcium, magnesium, iron, and aluminum. They form prismatic or needle-like crystals.
- Occurrence: These minerals account for about 7% of the Earth’s crust and are found in both igneous and metamorphic rocks.
- Uses: The diversity in the amphibole group means varied uses. Asbestos, a fibrous form of amphibole, was widely used for its fire-resistant properties before its health risks were fully acknowledged. Now, the use of asbestos is heavily regulated. Other amphibole minerals are studied for their geological significance.
Mica
- Properties: Mica refers to a group of silicate minerals that are layered or plate-like in structure and contain potassium, magnesium, aluminum, iron, and silica. They are known for their perfect cleavage, allowing them to be split into thin sheets.
- Occurrence: Constituting about 4% of the Earth’s crust, mica is found in igneous, metamorphic, and occasionally sedimentary rocks.
- Uses: Mica is widely used in the electrical industry as an insulator in capacitors and within cable insulation. Its heat-resistant properties make it valuable in electronics, and its reflective qualities are utilized in cosmetics.
Olivine
- Properties: Olivine is a group of silicate minerals composed mainly of magnesium, iron, and silica. It has a distinctive green color and is transparent to translucent.
- Occurrence: It is commonly found in basaltic rocks and is a major component of the Earth’s upper mantle.
- Uses: Besides its use as a refractory material due to its high melting point, olivine’s green color and clarity make it popular in jewelry. It is also being researched for its potential in sequestering carbon dioxide as a means of mitigating climate change.
Each of these minerals demonstrates the intricate relationship between Earth’s geological processes and human technology. From structural applications in construction to critical components in the tech and jewelry industries, these minerals underline the essential role geology plays in modern society.
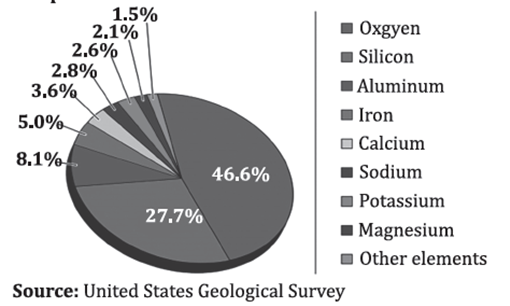
MAJOR ELEMENTS OF THE EARTH’S CRUST
The composition of the Earth’s crust is a fascinating topic within geology, offering insights into the Earth’s formation, structure, and the processes that shape its surface. The Earth’s crust is the outermost layer of our planet, lying above the mantle and is composed of a variety of elements, which are crucial in forming the rocks and minerals that make up the crust.
Major Elements of the Earth’s Crust
1. Oxygen (O)
- Abundance: The most abundant element in the Earth’s crust, constituting about 46.6% by weight.
- Role: Oxygen combines with other elements, especially silicon, to form silicates, which are the most common minerals in the Earth’s crust. Quartz (silicon dioxide, SiO2) is a common example.
2. Silicon (Si)
- Abundance: The second most abundant element, making up approximately 27.7% of the Earth’s crust by weight.
- Role: Silicon’s primary role is in forming silicates, the most prevalent group of minerals. Together with oxygen, it creates the framework for the silica-oxygen tetrahedra, fundamental to many rock-forming minerals.
3. Aluminium (Al)
- Abundance: Constituting about 8.1% of the crust by weight, aluminium is the third most abundant element.
- Role: Aluminium is a key component in many silicate minerals, including feldspars and micas, which are essential in forming igneous and metamorphic rocks.
4. Iron (Fe)
- Abundance: Although the most abundant element in the Earth as a whole due to its concentration in the core, it ranks fourth in the crust, making up about 5%.
- Role: Iron is a significant component of many minerals and rocks, imparting color and magnetic properties. It is crucial in forming ferromagnesian silicates and oxides.
5. Calcium (Ca)
- Abundance: Making up about 3.6% of the crust’s weight.
- Role: Calcium is a primary element in calcareous rocks (limestone and chalk) and is essential in the formation of silicate minerals like plagioclase feldspar.
6. Sodium (Na)
- Abundance: Comprises about 2.8% of the Earth’s crust.
- Role: Sodium is vital in forming alkali feldspar and plagioclase, which are significant components of many igneous rocks.
7. Potassium (K)
- Abundance: Accounts for about 2.6% of the crust by weight.
- Role: Potassium is a key element in forming alkali feldspars, which are important minerals in granitic rocks.
8. Magnesium (Mg)
- Abundance: Making up about 2.1% of the crust.
- Role: Magnesium is crucial in forming magnesium silicate minerals and is a major component of olivine, a mineral found in the Earth’s mantle and many igneous rocks.
Other Notable Elements
Besides the eight major elements, the Earth’s crust contains various other elements in smaller quantities, such as Titanium (Ti), Hydrogen (H), Phosphorus (P), Manganese (Mn), Sulphur (S), Carbon (C), and Nickel (Ni). These elements play significant roles in the composition and formation of specific minerals and contribute to the biochemical cycles and processes on Earth.
Rocks
Rocks, the solid foundation of Earth’s crust, are integral to understanding the planet’s composition, history, and the dynamic processes that shape its surface. Petrology, the scientific study of rocks, delves into their origins, composition, and relationships, providing insights crucial for various applications, from construction to mineral extraction.
1. Rocks
- Definition: Rocks are solid aggregates of minerals or mineraloids, occurring naturally.
- Variability: Their properties can significantly vary, covering the spectrum from hard to soft, and dark to light.
- Role and Importance: Rocks constitute the Earth’s crust, influencing the planet’s surface features while serving as vital resources for human civilization.
2. Petrology: The Study of Rocks
Petrology encompasses the study of rocks’ physical and chemical properties, their mineral content, texture, structure, and origin. This field is pivotal in understanding the Earth’s crust, aiding in the exploration of resources, and interpreting geological history.
3. Classification of Rocks
Rocks are categorized into three principal types based on their origin, each undergoing distinct processes:
4. Igneous Rocks
A. Formation:
These rocks form from the solidification of magma or lava, marking the inception of the rock cycle.
- Types:
- Intrusive (Plutonic) Rocks: Form below the Earth’s surface, cooling slowly to form large mineral grains. Examples include Granite, Diorite, and Gabbro.
- Extrusive Rocks: Emerge as lava on the Earth’s surface, cooling rapidly to create fine-grained textures. Basalt, particularly from the Deccan Traps of India, is a notable example.
B. Sedimentary Rocks
- Formation: These rocks arise from the accumulation, compaction, and cementation of sediment, or mineral precipitation from water, often layering and containing fossils.
C. Metamorphic Rocks
- Formation: Metamorphic rocks result from the transformation of existing rocks under high pressure and temperature, leading to a change in form without melting.
D. Igneous Rocks: A Closer Look
The study of igneous rocks reveals much about Earth’s interior processes:
- Texture: The texture varies from coarse-grained, indicating slow cooling beneath the Earth’s surface, to fine-grained, suggesting rapid cooling on the surface.
- Chemical Composition:
- Acidic Rocks: These rocks have high silica content, are light in color, and show high resistance to weathering. Examples include Granite and Quartz.
- Basic Rocks: Characterized by lower silica content, these rocks are darker and less resistant to weathering. Basalt and Gabbro are examples.
E. Significance of Igneous Rocks
Igneous rocks hold significant value for several reasons:
- Source of Minerals: They are the primary source of many metallic ores and precious minerals, including iron, copper, nickel, lead, zinc, and diamonds.
- Building Materials: Certain igneous rocks, like granite, are prized for their durability and aesthetic appeal in construction.
- Geological Insights: Features such as amygdales in basalt provide valuable information about the rock formation process and potential mineral deposits.
Sedimentary rocks
Sedimentary rocks represent a fundamental category of rocks formed from the deposition, compaction, and cementation of sediment. These processes collectively contribute to the lithification, or the transformation of loose sediment into solid rock. Understanding sedimentary rocks provides insight into Earth’s history, including the evolution of life and past climates. Here’s an expanded and detailed explanation:
Formation of Sedimentary Rocks
- Deposition and Compaction: Sedimentary rocks originate from the accumulation of material that has been eroded and transported by agents such as water, wind, and ice. Over time, these sediments are deposited in layers and gradually compacted under the weight of overlying materials.
- Lithification: This is the key process that turns sediments into solid rock. It involves two main steps: compaction, where the pressure from the layers of sediment squeezes out the water and air between the particles; and cementation, where minerals precipitate from groundwater and bind the sediment particles together.
- Volume vs. Crust Coverage: While sedimentary rocks constitute about 75% of the Earth’s surface, they only make up about 5% of the Earth’s volume. This discrepancy is due to their predominant location in the Earth’s crust, particularly on continents and continental shelves.
Characteristics of Sedimentary Rocks
- Layered Structure: The process of sediment deposition over time results in a distinctive layered appearance, known as stratification. This characteristic is a key indicator of sedimentary rocks.
- Fossils: Sedimentary rocks are the primary repository of fossils. The process of sediment deposition and subsequent lithification can preserve the remains of plants and animals, offering a window into past life on Earth.
- Permeability and Porosity: Many sedimentary rocks are permeable and porous, which means they can contain and transmit fluids. This characteristic makes them important reservoirs for groundwater and hydrocarbons.
Classification of Sedimentary Rocks
Sedimentary rocks are classified based on their mode of formation:
- Mechanically Formed Rocks: These are formed from the physical accumulation of sediment. Examples include:
- Arenaceous Rocks (e.g., sandstone): Characterized by larger particle sizes, these rocks are porous and typically hard.
- Argillaceous Rocks (e.g., shale): Composed of finer particles, these rocks are softer, impermeable, and less porous, making them more susceptible to weathering and erosion.
- Organically Formed Rocks: These originate from the accumulation of plant and animal remains that are subjected to pressure and temperature changes, leading to rock formation. Examples include:
- Calcareous Rocks (e.g., limestone, chalk): These rocks have a high calcium content.
- Carbonaceous Rocks (e.g., coal): These are rich in carbon content.
- Chemically Formed Rocks: Formation of these rocks occurs when minerals precipitate from a solution as water evaporates, leaving the mineral content to crystallize and form rocks. Examples include chert, potash, and halite.
Significance of Sedimentary Rocks
- Record of Earth’s History: Sedimentary rocks preserve the structure of ancient landscapes, climate data, and the history of erosion, offering invaluable insights into Earth’s past environments.
- Evolutionary Evidence: The fossils found within sedimentary layers serve as evidence of the evolution of life, documenting changes in flora and fauna over millions of years.
- Resource Reservoirs: Sedimentary rocks are significant sources of natural resources, including energy minerals like coal and shale gas, making them crucial for understanding and exploiting Earth’s natural wealth.
METAMORPHIC ROCKS
Metamorphic rocks represent a fascinating branch of geology, showcasing how the forces of nature can transform materials over time. Understanding these rocks provides insights into the Earth’s dynamic processes and the conditions beneath its surface.
Formation of Metamorphic Rocks
Metamorphic rocks originate from the transformation of pre-existing rocks, whether igneous, sedimentary, or even older metamorphic types. This transformation, known as metamorphism, occurs under variations in pressure, volume, and temperature (PVT), without the rock melting into magma. The process involves two key mechanisms:
- Recrystallization: The minerals within the original rock undergo changes in size and shape but remain solid, leading to new mineral assemblages that are stable under the new PVT conditions.
- Reorganization: The texture and structure of the rock are altered, often leading to the development of foliation or a layered appearance.
Characteristics of Metamorphic Rocks
Metamorphic rocks exhibit a range of distinctive features:
- Durability: They are generally hard and resistant to erosion and weathering, making them structurally robust.
- Varied Colors: These rocks can display a wide spectrum of colors, depending on the minerals present.
- Banding: Some metamorphic rocks show banded structures, especially in foliated types like gneiss and schist.
- Fossil Rarity: Due to the extreme conditions of their formation, metamorphic rocks rarely contain fossils.
- Chemical Reactivity: Certain metamorphic rocks react with acids, a characteristic influenced by their mineral composition.
Processes of Metamorphism
Metamorphism can occur through several processes, primarily dynamic and thermal metamorphism:
- Dynamic Metamorphism: This process involves the physical restructuring and recrystallization of rocks under pressure, typically without significant chemical changes. It often occurs in areas of intense tectonic activity, where rocks are deformed by folding and faulting.
- Thermal (Contact) Metamorphism: Here, rocks are altered by high temperatures, usually when in contact with magma. This can lead to the formation of new minerals and textures.
- Regional Metamorphism: Occurs over broad areas under the combined influence of high pressure and temperature, often related to tectonic processes such as continental collision.
Types of Metamorphic Rocks
Metamorphic rocks are classified based on their texture, particularly into foliated and non-foliated types:
- Foliated Metamorphic Rocks: These have a layered or banded appearance due to the parallel alignment of mineral grains. Examples include slate, schist, phyllite, and gneiss.
- Non-Foliated Metamorphic Rocks: These rocks do not exhibit a layered texture. Their formation is typically influenced by conditions where pressure is applied equally from all directions, or where the rock is composed of minerals that do not form layers. Examples are marble and quartzite.
Significance of Metamorphic Rocks
Metamorphic rocks have various uses and significances:
- Building Materials: Slate and marble are prized for their beauty and durability in construction and sculpture.
- Industrial Applications: Talc is widely used in cosmetics and paints, garnet as an abrasive, and asbestos for its fireproofing and insulating properties.
Examples of Metamorphic Transformations
The table below illustrates how different types of rocks can metamorphose into metamorphic rocks:
| Type of Rock | Original Rock | Metamorphic Rock |
| Igneous | Granite | Gneiss |
| Igneous | Basalt | Hornblende |
| Sedimentary | Limestone | Marble |
| Sedimentary | Coal | Graphite |
| Sedimentary | Sandstone | Quartzite |
| Sedimentary | Shale/Clay | Slate/Mica Sheet |
This transformation showcases the dynamic and cyclical nature of geological processes, where even the oldest rocks can be reshaped into new forms, contributing to the ever-changing landscape of our planet. Metamorphic rocks thus serve as a testament to the Earth’s internal energies and the complex interactions between its various layers.
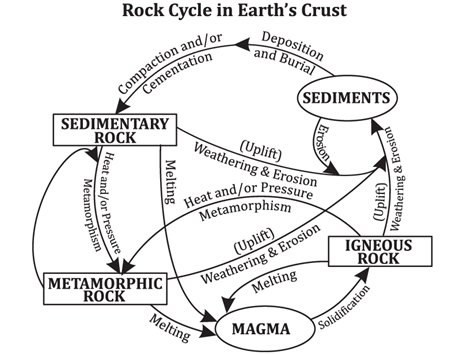
Rock cycle
The rock cycle is a fundamental concept in geology that describes the dynamic and continuous process through which rocks are formed, broken down, and transformed over geological time scales. This cycle is driven by various geological processes, including plate tectonics, weathering, erosion, and sedimentation.
1. Formation of Igneous Rock
Igneous rocks are formed from the solidification of molten rock material. There are two primary settings where this occurs:
- Volcanic (Extrusive) Igneous Rocks: These are formed from lava that oozes out onto the Earth’s surface, where it cools and solidifies quickly. Due to the rapid cooling, these rocks often have fine-grained or glassy textures.
- Plutonic (Intrusive) Igneous Rocks: These rocks form from magma that solidifies slowly beneath the Earth’s surface. The slow cooling allows for the growth of larger crystals, resulting in a coarse-grained texture.
2. Formation of Sedimentary Rock
Sedimentary rocks are formed from the accumulation and lithification of sediment. This process involves several stages:
- Weathering: The breakdown of rocks and minerals into smaller pieces by physical, chemical, or biological means.
- Erosion: The removal and transportation of sediment by natural agents such as water, wind, ice, or gravity.
- Deposition: The accumulation of sediments in layers at the Earth’s surface or within bodies of water.
- Compaction and Cementation: Over time, accumulated sediments are buried under additional layers, compacting them. Mineral-rich water flows through the sediments, cementing them together to form solid rock.
3. Formation of Metamorphic Rock
Metamorphic rocks are formed from the transformation of existing rock types (igneous, sedimentary, or other metamorphic rocks) in a process called metamorphism. This transformation occurs under conditions of elevated temperature and pressure, which can alter the mineralogical and structural composition of the rock without melting it. There are two main types of metamorphism:
- Regional Metamorphism: Occurs over large areas and is typically associated with mountain-building processes.
- Contact Metamorphism: Occurs when rock is heated by the intrusion of hot magma from the Earth’s interior.
4. Weathering
Weathering is the process by which rocks are broken down at the Earth’s surface, contributing to the formation of sediment. Weathering can be physical (breaking rocks into smaller pieces), chemical (altering minerals within rocks), or biological (involvement of living organisms).
5. Transportation
The sediments produced by weathering are transported by natural agents like water, wind, and ice. The distance and method of transportation can affect the size, sorting, and composition of the sediments.
6. Deposition and Sedimentation
Deposition is the process by which transported sediments are laid down or settle in a new location. Over time, these sediments can accumulate in layers, which may eventually become compacted and cemented to form sedimentary rocks.
The Continuous Cycle
The rock cycle is a continuous process. Sedimentary rocks can be buried and subjected to high temperature and pressure, transforming them into metamorphic rocks. Metamorphic rocks, if melted, become magma, which can cool to form igneous rocks. Similarly, igneous rocks can be weathered and eroded to form sediments, which can then become sedimentary rocks. This cycle illustrates the dynamic nature of Earth’s crust and how geological processes are interconnected.
UPSC PREVIOUS YEAR QUESTIONS
1. Consider the following: (2013)
1. Electromagnetic radiation
2. Geothermal energy
3. Gravitational force
4. Plate movements
5. Rotation of the earth
6. Revolution of the earth
Which of the above are responsible for bringing dynamic changes on the surface of the earth?
(a) 1, 2, 3 and 4 only
(b) 1, 3, 5 and 6 only
(c) 2, 4, 5 and 6 only
(d) 1, 2, 3, 4, 5 and 6
2. Consider the following statements: (2013)
1. Natural gas occurs in the Gondwana beds.
2. Mica occurs in abundance in Kodarma.
3. Dharwars are famous for petroleum.
Which of the statements given above is/are correct?
(a) 1 and 2
(b) 2 only
(c) 2 and 3
(d) None
3. Which of the following adds nitrogen to the soil? (2013)
1. Excretion of urea by animals
2. Burning of coal by man
3. Death of vegetation
Select the correct answer using the codes given below:
(a) 1 only
(b) 2 and 3 only
(c) 1 and 3 only
(d) 1, 2 and 3
4. With reference to the management of minor minerals in India, consider the following statements: (2019)
1. Sand is a ‘minor mineral’ according to the prevailing law in the country.
2. State Governments have the power to grant mining leases of minor minerals, but the powers regarding the formation of rules related to the grant of minor minerals lie with the Central Government.
3. The State Government has the power to frame rules to prevent illegal mining of minor minerals.
Which of the statements given above is/are correct?
(a) 1 and 3 only
(b) 2 and 3 only
(c) 3 only
(d) 1, 2 and 3
5. Consider the following minerals: (2020)
1. Bentonite
2. Chromite
3. Kyanite
4. Sillimanite
In India, which of the above is/are officially designated as major minerals?
(a) 1 and 2 only
(b) 4 only
(c) 1 and 3 only
(d) 2, 3 and 4 only