UNDERSTANDING ACID RAIN
Origins of Acid Rain:
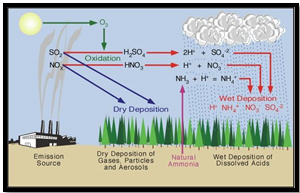
- Acid rain results from the combustion of fossil fuels containing sulphur, releasing sulphur dioxide (SO2), and nitrogen oxide (NOx) emissions, particularly from vehicles and power plants.
- Natural sources like volcanic eruptions and lightning also contribute to SO2 and NOx emissions, but urban areas rely heavily on fossil fuels, exacerbating the issue.
Source: Weather Website
Formation and Effects:
- SO2 and NOx react with water and oxygen in the atmosphere to form sulphuric acid (H2SO4) and nitric acid (HNO3), leading to acid rain, snow, and fog with a pH around 4.2-4.4.
- Acid precipitation can harm aquatic life by rendering water bodies inhospitable and disrupt soil bacteria, impacting ecosystems and forests.
Mitigation Efforts:
- Coal power plants have reduced SO2 emissions significantly using flue-gas desulphurisation, while international collaborations like the Acid Deposition Monitoring Network in East Asia (EANET) aim to minimize acid rain’s environmental impact.

 Source: Weather Website
Source: Weather Website