Helium and Its Role in Rockets
Why in the news?
Helium issues caused delays in SpaceX’s Polaris Dawn mission and affected NASA’s Starliner and other missions. The gas’s crucial role in rocket fuel systems and its leakage problems highlight industry challenges.
Why Helium is Used in Rockets:
- Helium is inert (non-reactive) and lightweight, with an atomic number of 2, making it the second lightest element after hydrogen.
- It doesn’t react with other substances or combust, crucial for maintaining rocket stability.
- Helium’s low boiling point (-268.9°C) allows it to remain gaseous in extremely cold environments, which is essential since many rocket fuels are stored at such temperatures.
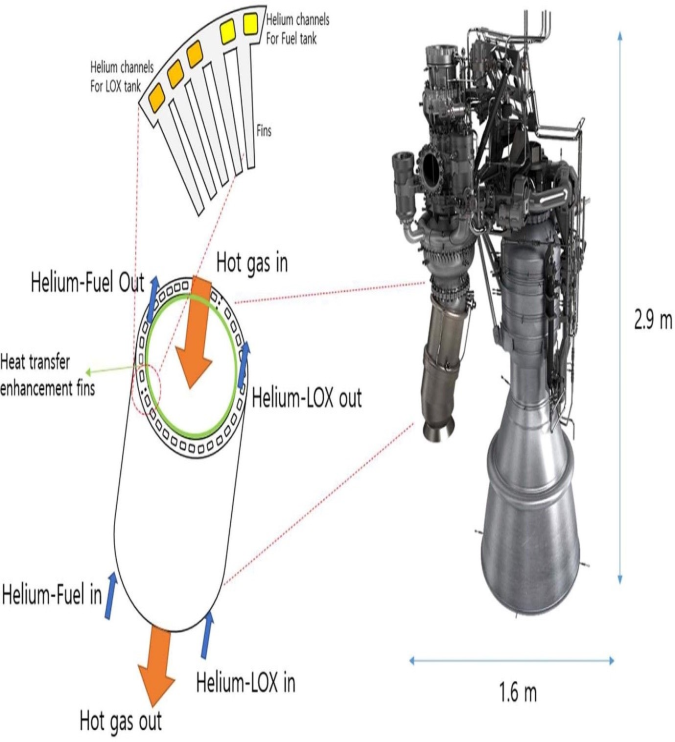
Functions in Spacecraft:
- Helium pressurised fuel tanks, ensuring uninterrupted fuel flow to engines.
- It fills the empty spaces in tanks as fuel burns, maintaining overall tank pressure.
- As it’s non-reactive, helium can safely interact with residual fuel and oxidizer in the tanks, preventing hazards.
Challenges and Leakage Issues:
- Helium’s small atomic size and low molecular weight make it prone to leaks through small gaps or seals.
- While leaks can occur, helium’s scarcity in Earth’s atmosphere makes detecting them easier, aiding in fault identification in rocket systems.
- Frequent helium leaks in missions (e.g., NASA’s Starliner, SpaceX’s Polaris Dawn, ISRO’s Chandrayaan-2) highlight the need for improved valve design and tightening mechanisms in the space industry.
About Helium:
- Symbol: He, Atomic Number: 2, Atomic Mass: 4
- Group: 18 (Noble Gases), State at 20°C: Gas
- Discovered in 1895 by Sir William Ramsay, Per Teodor Cleve, and Nils Abraham Langlet
- Named after “Helios” (Greek for Sun), first detected in the Sun’s corona
Key Properties
- Inert, colourless, and odourless gas
- Density: 000164 g/cm³, Boiling Point: 4.222 K
- 2nd most abundant element in the universe
- Extracted from natural gas (up to 7% concentration)
Uses and Role in Rocket Launches:
- Fuel Tank Pressurisation: Helium ensures steady fuel flow by maintaining pressure in emptied tanks
- Inert Nature: Used in highly reactive fuel systems (liquid oxygen/hydrogen) to prevent contamination
- Low Boiling Point: Ideal for cryogenic fuel storage systems
- Leak Detection: Helium’s small atomic size helps engineers identify fuel system leaks
- Cooling Systems: Cools rocket components, including sensitive electronics and propulsion systems
- Additional uses: Deep-sea diving, airships, MRI scanners, and in semiconductor manufacturing.
Sources Referred:
PIB, The Hindu, Indian Express, Hindustan Times