Ammonia Production: Key to Agriculture And Environmental Challenges
Syllabus:
GS-2: Conservation
GS-3: Government Policies & Interventions
Focus:
Ammonia production is under scrutiny due to its environmental impact. The Haber-Bosch process, while pivotal in increasing food supply, raises concerns about excessive nitrogen use, contributing to soil degradation and water pollution. Discussions on sustainable fertiliser practices and regulatory frameworks are gaining prominence as global food demand rises.
Introduction to Ammonia Production:
- Chemical Composition: Ammonia (NH₃) is produced from nitrogen (N₂) and hydrogen (H₂).
- High-Temperature Reaction: Under extreme heat, N₂ and H₂ molecules separate and attempt to form ammonia, but the compound is unstable at high temperatures.
- Fritz Haber’s Innovations: German chemist Fritz Haber pioneered the process by heating the nitrogen-hydrogen mixture in a platinum cylinder and applying pressure to synthesise ammonia.
Key Facts About Ammonia:
- Appearance: Ammonia is a colourless gas with a sharp, suffocating odour.
- Solubility: It dissolves easily in water, forming ammonium hydroxide, which can cause irritation and burns.
- Compression: Ammonia gas can be easily compressed into a clear, colourless liquid under pressure.
- Shipping: It is typically transported as a compressed liquid in steel cylinders.
- Flammability: Ammonia is not highly flammable, but it’s containers can explode if exposed to high heat.
What is Green Ammonia?
- Definition: Green ammonia is produced using 100% renewable and carbon-free processes.
- Production Method: It utilises hydrogen from water electrolysis and nitrogen separated from the air, all powered by sustainable electricity.
- Current Production: Traditional ammonia is mainly made from methane via steam methane reforming (SMR), resulting in high carbon dioxide
- Decarbonization: Transitioning to low-carbon hydrogen (blue or green) is essential for reducing emissions in ammonia production.
- Energy Storage: Ammonia can be stored in bulk as a liquid, making it an ideal chemical store for renewable energy.
- Zero-Carbon Fuel: When burned or used in fuel cells, ammonia produces only water and nitrogen as by-products.
- Hydrogen Carrier: Ammonia is easier and cheaper to store and transport than hydrogen, allowing for efficient conversion back to hydrogen gas when needed.
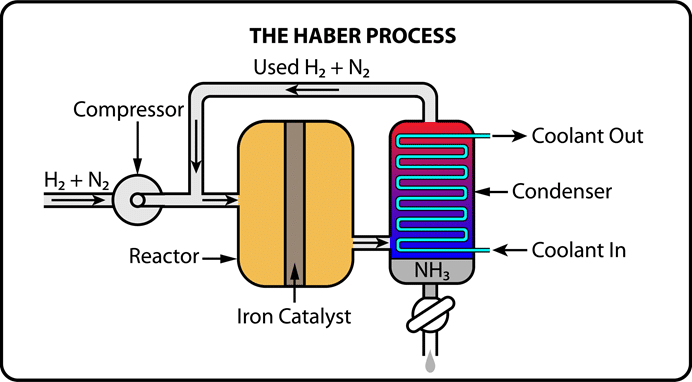
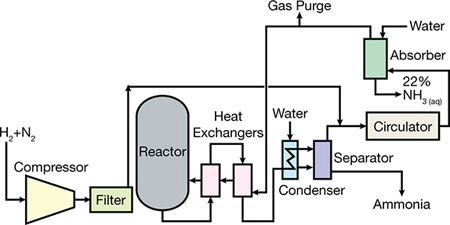
Key Points about the Haber-Bosch Process:
- Definition: The Haber-Bosch process is an industrial method for producing ammonia efficiently.
- Developers: Initiated by Fritz Haber; industrialised by Carl Bosch in
- Raw Materials: Utilises nitrogen (from the atmosphere) and hydrogen (from natural gas).
- Reaction: The balanced equation is N2(g)+3H2(g)→2NH3(g)N_2(g) + 3H_2(g) \rightarrow 2NH_3(g)N2(g)+3H2(g)→2NH3(g).
- Conditions: Operates at high pressures (200-400 atm) and temperatures (~500°C).
- Catalyst: Iron catalyst, often combined with potassium hydroxide, enhances reaction efficiency.
- Hydrogen Source: Commonly obtained through steam methane reforming (from natural gas).
The Haber-Bosch Process: Transforming Agriculture
- Historical Impact: The Haber-Bosch process enabled the production of affordable synthetic fertilisers, contributing to a sevenfold increase in the global food supply during the 20th century.
- Fertilizer Use and Environmental Concerns: While fertilisers have significantly enhanced food production, environmentalists warn that their benefits should not be taken for granted. The fertilisers can lead to ecological issues if used excessively.
- Current Statistics: The Haber-Bosch process facilitates the removal of over 100 million tonnes of nitrogen from the atmosphere annually, adding approximately 165 million tonnes of reactive nitrogen to the soil, surpassing natural replenishment methods.
Understanding Nitrogen and Its Sources:
- Nitrogen Molecule Structure: Nitrogen molecules (N₂) consist of two nitrogen atoms bonded by a strong triple bond, making them inert and difficult to break without significant energy.
- Natural Nitrogen Sources: Natural processes, such as lightning, can break the N₂ bond, forming reactive nitrogen compounds like nitrogen oxides (NO and NO₂), which are then converted into nitrates and ammonium through rainfall.
- Microbial Nitrogen Fixation: Certain bacteria, such as Azotobacter, and plants like legumes form symbiotic relationships that help convert atmospheric nitrogen into reactive forms usable by plants, replenishing soil nitrogen levels.
The Nitrogen Cycle and Agricultural Dependency:
- Nitrogen Absorption in Plants: Plants typically absorb reactive nitrogen in the form of ammonium (NH₄⁺) and nitrate (NO₃⁻) from the soil.
- Human and Animal Nutrition: Humans and animals obtain essential amino acids by consuming plants, which incorporate nitrogen into their structures.
- Nitrogen Return to Soil: Nitrogen cycles back into the soil through animal excretion and the decomposition of organic matter, although some is lost back to the atmosphere as molecular nitrogen (N₂).
The Technical Aspects of Ammonia Synthesis:
- Haber’s Early Work: Fritz Haber’s experiments involved heating N₂ and H₂ mixtures in a platinum cylinder to produce ammonia.
- His initial attempts produced negligible amounts for commercial viability.
- Catalysts and Pressure: Realising that higher pressures (around 200 atm) and temperatures (approximately 200°C) could enhance production, Haber sought catalysts to increase reaction efficiency.
- Collaborative Innovations: The process was further refined with the contributions of engineers like Robert Le Rossignol and Friedrich Kirchenbauer, who built pressure-resistant chambers to facilitate the synthesis.
Major Challenges Associated with Using Ammonia as a Fuel:
- Environmental Impact: While ammonia combustion has near-zero CO2 emissions, it still produces unburned ammonia and nitrogen oxides (NOx), contributing to air pollution, respiratory illnesses, and acid rain.
- Production Challenges: The traditional Haber-Bosch process for ammonia production is energy-intensive and relies on fossil fuels.
- Green ammonia production using renewable energy is still developing and faces high costs and scalability issues.
- Toxicity: Ammonia is highly toxic and poses serious health risks if not managed properly.
- Its corrosiveness can lead to severe consequences in case of accidents or mishandling.
- Fuel Quality Standards: Establishing consistent quality standards for ammonia as fuel is complex, particularly when it is sourced from diverse methods and may contain various impurities.
- Infrastructure Limitations: Current infrastructure may not be equipped to handle ammonia safely or efficiently, requiring significant investment in new facilities and distribution systems.
- Public Perception: Concerns regarding the safety and toxicity of ammonia fuel can hinder its acceptance and adoption in various sectors.
- Regulatory Challenges: Existing regulations may not adequately address the specific needs and risks associated with using ammonia as a fuel, necessitating updates to ensure safety and environmental protection.
Way Forward:
- Improved Engine Technology: Investing in research to develop more efficient ammonia engines with optimised combustion processes can minimise NOx emissions and improve performance.
- Safety Training: Implementing comprehensive training programs for ammonia industry workers will ensure proper handling, safety protocols, and emergency response practices, reducing risks.
- Market Incentives: Creating incentives, such as tax credits or subsidies, can encourage the adoption of ammonia as a fuel, particularly in high-impact sectors like maritime transport.
- Ammonia Hybrids: Developing hybrid systems that integrate ammonia with renewable energy sources (e.g., solar and wind) can enhance energy stability and utility during low generation periods.
- Research in Green Ammonia Production: Investing in R&D for green ammonia production methods can help lower costs and improve scalability, making ammonia a more sustainable fuel option.
- Infrastructure Development: Upgrading existing infrastructure and developing new facilities designed for ammonia handling will ensure safe and efficient distribution.
- Regulatory Framework: Establishing a clear regulatory framework tailored to the unique properties and risks associated with ammonia fuel will promote safety, environmental protection, and industry growth.
Environmental Consequences and Future Outlook:
- Fertiliser Dependency and Nutrient Imbalance: Over-application of synthetic fertilisers can lead to nutrient imbalances in soils, as they draw more nitrogen from the earth than it can naturally replenish.
- Environmental Impact: Excess nitrogen can cause problems like water pollution, algal blooms, and soil degradation. It contributes to the acidification of water bodies and loss of biodiversity.
- The Need for Balanced Solutions: The lesson from nitrogen fixation is that technological solutions like the Haber-Bosch process must be complemented with political action and societal mobilisation to address food security and environmental sustainability challenges effectively.
Conclusion:
The Haber-Bosch process revolutionised agriculture by enabling large-scale ammonia production, crucial for synthetic fertilisers. However, its environmental repercussions highlight the need for sustainable practices. Addressing nitrogen pollution through regulation, education, and innovative farming methods will be essential for balancing agricultural productivity with ecological health, ensuring food security for future generations.
Source: The Hindu
Mains Practice Question:
Discuss the significance of the Haber-Bosch process in modern agriculture. What are the environmental concerns associated with ammonia production, and how can sustainable practices mitigate these issues? Provide specific examples of alternative nitrogen sources and farming techniques that can enhance soil health while maintaining crop productivity.