Gene Therapy Successfully Treats Haemophilia A in India
Why in the news?
Indian scientists have made significant advancements in using gene therapy to treat Haemophilia A, a rare blood disorder. This breakthrough offers long-term relief by addressing the genetic cause, improving quality of life for affected individuals.
About Gene Therapy Breakthrough for Haemophilia A in India:
- Indian scientists have achieved significant progress in gene therapy for treating severe Haemophilia A, a rare inherited disorder where the blood lacks clotting factor VIII, crucial for blood clot formation.
- Haemophilia A is a sex-linked condition carried on the X chromosome, leading to impaired coagulation and prolonged bleeding.
Traditional Treatment and Limitations
- Conventional treatment for Haemophilia A involves clotting factor replacement therapy to boost clotting factor levels or medications to encourage clotting.
- While effective, these treatments require regular administration and don’t address the root cause of the disorder.
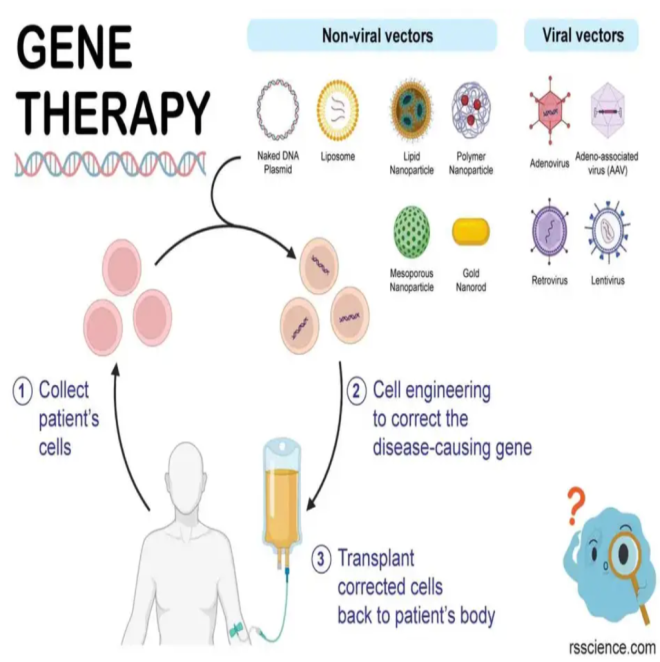
Gene Therapy: A Revolutionary Approach
- Gene therapy aims to correct the genetic cause of Haemophilia A by introducing functional genes that enable the body to produce the missing clotting factor.
- This method reduces dependency on regular treatments and promises better quality of life for those affected.
- The success of gene therapy in India marks a transformative step, potentially offering long-term relief for individuals with Haemophilia A, both in India and globally.
About Haemophilia A:
- Cause: Hemophilia A is caused by mutations in the F8 gene on the X chromosome, leading to insufficient factor VIII, a clotting protein.
- Prevalence: More common in males, though females can have mild symptoms if they inherit one defective gene.
Gene Therapy for Haemophilia:
- Treatment: Gene therapy (e.g., Roctavian) introduces a therapeutic gene via an adenovirus vector, enabling the liver to produce factor VIII.
- FDA Approval: Roctavian was approved by the U.S. FDA for commercial use in 2023.
Related Government Initiatives:
- India Semiconductor Mission: Aims to boost semiconductor manufacturing in India for self-reliance in electronics.
- Artificial Intelligence Mission: Focuses on AI innovation and integration in various sectors.
- Unified Payment Interface (UPI): Facilitates seamless, instant digital payments.
- INS Vikrant: India’s first indigenous aircraft carrier, enhancing naval capabilities.
- Bharat 6G Project: Develops next-generation 6G technology for India’s telecom sector.
- India-US Critical & Emerging Technology Initiative: Strengthens collaboration in advanced technologies between India and the US.
Sources Referred:
PIB, The Hindu, Indian Express, Hindustan Times