Why arrival of Mpox vaccines was delayed in Africa
Relevance: GS – 3 : Science and Technology – developments.
Why in the news?
- The Democratic Republic of Congo (DRC), the epicenter of the outbreak of the mpox epidemic, received its first vaccinations.
- The epidemic that began in the DRC has spread to other countries and intensified.
- The vaccination came about a month after the World Health Organization (WHO) declared mpox a global health emergency.
- Vaccination delays are cause for concern, especially when the virus has become more virulent.
- The DRC received the vaccines as part of an international philanthropic effort.
More about the news:
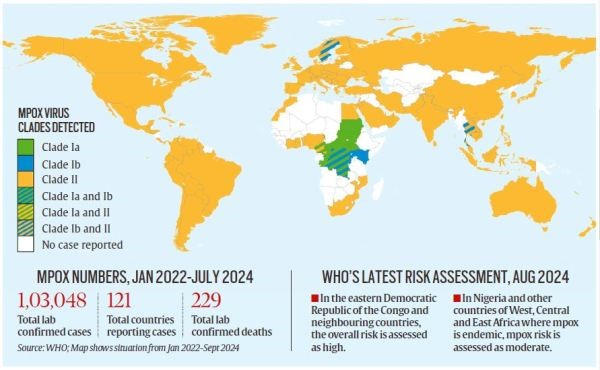
- As of January 1, 2022, mpox has been reported in 121 countries out of 20 WHO member states in Africa.
- As of September 5, 2024, there have been 103,048 laboratory cases of mpox worldwide, with 229 deaths reported.
- India on Monday confirmed its first case of mpox, a travel-related virus.
- Despite increasing cases, there is a severe shortage of mpox vaccines in Africa.
Apart from the DRC, Nigeria is the only other African country that has received mpox vaccines. - A lack of vaccines in Africa has led to the rapid spread of mpox, prompting the WHO to declare the virus a global health emergency last month.
Reasons for concern about Mpox Spread
- First reported in humans in the 1970s, Mpox has gained attention due to the widespread distribution of another Clade Ib species.
- Clade characteristics: There are two known clades of mpox – Clade I and Clade II. The first clade is believed to have killed the two.
- Clade Ib Messages: Clade Ib appears to move between humans relatively rapidly, including through sexual activity. In contrast, clade Ia is largely derived from animals.
- Number of people affected: The new Clade Ib is affecting many women and children in African countries. Scientists are still sorting out the mechanisms that make Clade Ib’s transmit rapidly from person to person.
- Smallpox and mpox both affect the same genus of viruses (Orthopoxvirus).
- Mpox is less severe than smallpox and has a lower mortality rate.
- But mpox spreads quickly because it contains animal reservoirs, a feature that smallpox lacked, allowing it to persist and spread quickly.
Mpox vaccine
There are three currently available mpox vaccines, all based on attenuated versions of the vaccinia virus, which were also used to make smallpox vaccines.
1. Modified Vaccinia Ankara (MVA):
- Manufacturer: Bavaria Nordic (Denmark).
- Approved by: U.S. FDA and European Medicines Agency (EMA).
- A common vaccine, was obtained by the Democratic Republic of the Congo (DRC).
2. LC16m8:
- Manufacturer: KM Biologics (Japan).
- Approval: For the exclusive use of mpox by the Japanese Legislative Assembly.
3. ACAM2000:
- Manufacturer: Emergent BioSolutions (US).
- Recently, the U.S. FDA approved mpox
Development of new vaccines:
- BioNTech (Germany) is developing a new mpox vaccine.
- The Indian Serum Institute in Pune is also working on the vaccine, aiming to achieve positive results within a year.
- The Indian Council of Medical Research (ICMR) has invited pharmaceutical companies and research institutes to collaborate on developing a mpox vaccine and diagnostic kit.
Reasons for delays in vaccine distribution in Africa
- High cost: Mpox vaccines are expensive, costing between $50 and $75 per dose, making them unaffordable for many African countries.
- Reliance on donations: African countries often rely on donations from developed countries and vaccine manufacturers, as well as Global Alliance for Vaccines and Immunization (Gavi, the Vaccine Alliance) and UNICEF-sponsored procurement.
- WHO approval: Gavi and UNICEF cannot procure vaccines until WHO issues emergency supplies or full approval. Companies must provide safety and efficacy data for this process.
- Delays in approvals: Some experts believe the WHO has been slow to issue these approvals, but WHO Director-General Tedros Adhanom Ghebreyesus attributed the delays to agencies failing to complete the paperwork.
- Legislative reliance: High-income countries rely on their drug regulators, and low-income and middle-income countries rely on WHO approval, which is often slow.
- Past infections: Low rates in past mpox have prevented many African countries from asking for vaccines to focus on other pressing health challenges
- Prevention programs have been suspended: Experts like Raman Gangakhedkar argue that the WHO should have introduced prolonged smallpox vaccination sooner to prevent the spread of the more virulent forms of mpox and to address gaps in vaccination coverage.
When to take Mpox vaccine
- High-risk populations: Vaccination is recommended for high-risk individuals, especially during outbreaks.
- Vaccination after exposure:
○ If a person has been in contact with someone who has pox, the vaccine should be administered within four days of exposure to pox.
○ In the absence of symptoms, it can be continued for up to 14 days after exposure.
Alternative articles
https://universalinstitutions.com/india-reports-first-suspected-mpox-case-in-isolation/
https://universalinstitutions.com/mpox-outbreak-a-global-health-concern-with-implications-for-india/
https://universalinstitutions.com/who-launches-strategic-plan-to-combat-mpox-outbreaks/
Source: https://indianexpress.com/article/explained/explained-health/monkey-pox-mpox-vaccines-donation-democratic-republic-of-congo-mpox-vaccines-origin-9559205/
Mains question
Discuss the challenges faced by African nations in accessing mpox vaccines and the role of international organizations in addressing vaccine inequity during global health emergencies. (250 words)