TR1 Cells Hold Key to Malaria Reinfection Immunity
TR1 Cells Hold Key to Malaria Reinfection Immunity
Why in the News?
A major study published in Science Immunology has identified T regulatory 1 cells (TR1) as crucial in the cellular immune response to malaria. This discovery in malaria immunology could revolutionize malaria vaccine research and immune-based therapies for malaria and other infectious diseases with no effective vaccines yet. The study sheds light on T cell activation in malaria and the role of TR1 cells in malaria immunity.
Understanding the Immune Response to Malaria:
- The human immune system uses innate and adaptive immunity against malaria to fight infections.
- Adaptive immunity, involving B-cells and T-cells, retains memory cells that respond faster on reinfection, contributing to long-term protection against malaria.
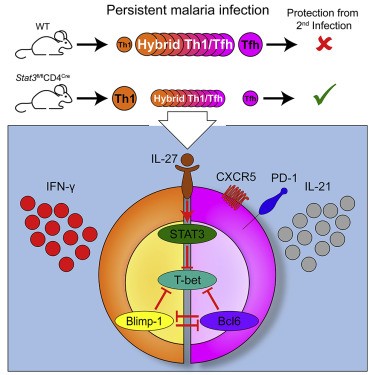
- The study focused on CD4+ T cells, especially TR1 cells, previously underexplored in T cell-mediated immunity.
- TR1 cells help activate B-cells, T-cells, and macrophages, making them central to immune coordination and malaria antigen presentation. This process involves T cell activation in malaria and immune effector expression.
Key Findings from Uganda-Based Longitudinal Study
- Conducted in eastern Uganda, where Plasmodium falciparum is widespread.
- Used single-cell RNA sequencing and TCR tracking to monitor CD4+ T-cell clones across repeated infections.
- TR1 cells, although only 3% of resting CD4+ T cells, formed 90% of Pf-specific helper cells, demonstrating significant clonal expansion and proliferation in malaria.
- TR1 cells showed high clonal fidelity, long-term memory, and antigen-specific activation during reinfection, key factors in malaria reinfection prevention.
- Th1 cells, once believed dominant, did not expand upon reinfection, indicating they are not Pf-specific in Th1 recall responses.
Implications for Vaccine and Immunotherapy Development
- TR1 cells’ proliferation correlates with malaria parasite load and persists after recovery, suggesting their role in immunological memory and malaria.
- Their unique gene expression dynamics and epigenetic regulation suggest they can be targeted for host-directed therapies.
- Opens doors to next-gen malaria vaccine candidates and improved treatments for malaria and other infections lacking effective vaccines.
About TR1 Cells:
- Type-1 regulatory T-cells (TR1) are a subset of CD4+ T cells.
- They regulate immune responses and prevent excessive inflammation.
- In malaria, TR1 cells dominate the antigen-specific immune response.
- They show high clonal fidelity and long-term memory.
- Their role could aid malaria vaccine efficacy and immunotherapy development.
CD4+ T-Cells:
- CD4+ T cells, or helper T-cells, orchestrate immune responses.
- They activate B-cells, CD8+ T-cells, and macrophages.
- Include subtypes like TR1, Th1, and Th2 cells.
- In malaria, TR1 cells emerged as dominant over Th1 in T cell polarization.
- Critical in shaping adaptive immunity against malaria and memory.
Malaria Reinfection
- Malaria reinfection occurs in endemic regions, often multiple times yearly.
- Individuals develop clinical immunity—resistance to symptoms, not infection.
- Reinfections help shape a robust immune memory, especially via TR1 cells.
- Reinfections were studied to identify long-lasting immune responses.
- Insights inform targeted vaccine strategies for malaria vaccine candidates.
Plasmodium falciparum:
- P. falciparum is the most deadly malaria parasite species.
- Transmitted by the female Anopheles mosquito.
- Causes severe disease, especially in young children in Africa.
- Main target of the study revealing TR1-mediated immunity.
- Drives malaria-related mortality and reinfection rates globally.
Vaccine Development:
- Vaccines train the immune system to fight infections.
- For malaria, progress has been slow due to complex parasite biology.
- TR1 cell findings offer a new target for effective malaria vaccine design.
- Long-term immunity and antigen specificity are key features.
- May inspire next-gen vaccines targeting malaria parasite antigens.
T-cell Sequencing:
- Single-cell RNA sequencing and TCR tracking helps map immune cell behavior.
- Used to track CD4+ T-cell clones in malaria patients.
- Reveals gene expression, antigen specificity, and memory traits.
- Critical in identifying dominant TR1 cell responses.
- Aids precision immunology research and spatial transcriptomics.
Clonal Fidelity:
- Refers to the accuracy with which immune cells replicate during response.
- High clonal fidelity means better memory and targeted immunity.
- TR1 cells showed strong clonal fidelity across infections.
- Ensures sustained, antigen-specific responses over time.
- Key trait for durable vaccine design and memory persistence.
Additional Insights:
- GC Tfh cells play a crucial role in B cell follicles during malaria infection. These GC Tfh cells contribute to antibody production.
- IL-10 production by TR1 cells helps regulate the immune response.
- TCM cells contribute to long-term protection against malaria.
- CXCR6 upregulation in TR1 cells may enhance their tissue-homing capabilities.
- Heterogeneous responses among CD4+ T cells highlight the complexity of malaria-specific T cells.
- Effector expansion and subset-specific activation are key processes in the CD4+ T-cell response to malaria.
- Transcriptional states of TR1 cells reveal unique patterns of gene expression dynamics.
- Longitudinal tracking of T cell responses provides insights into memory T cells in malaria.
- The study’s findings may lead to improved strategies for malaria reinfection prevention.
This groundbreaking research in T cell-mediated immunity and malaria immunology opens new avenues for malaria vaccine research and the development of more effective malaria vaccine candidates. By understanding the role of TR1 cells in the cellular immune response to malaria, scientists are one step closer to achieving long-term protection against malaria and potentially other challenging infectious diseases.
The study highlights the importance of CD4+ T cells in the immune response to Plasmodium falciparum, particularly the role of TR1 cells in malaria immunity. The research reveals intricate details about T cell activation in malaria, including the proliferation of specific cell types and the process of T cell polarization. The identification of antigen-specific clones and their immune effector expression provides valuable insights for future vaccine development.
Furthermore, the study sheds light on the dynamics within T cell zones and the importance of IFN-γ secretion in the immune response to malaria. These findings, combined with the understanding of TR1 cells in malaria immunity, pave the way for more targeted and effective approaches in combating this persistent global health challenge.