The Photoelectric effect: Drawing of a century old discovery
Syllabus:
GS 3:
- Science and technology and its applications in everyday life.
- Development of Technology
Focus:
A recent study on the photoelectric effect, the implementation of attosecond light pulses brings fresh knowledge in the field. At the SLAC National Accelerator Laboratory, researchers observed large time delays in electron emissions. This observation provided better understanding of the molecular and electronic structures. These structures are vital to future fundamental electronics and biochemical imaging.
Origins of the Photoelectric Effect:
- Einstein’s Contribution: Albert Einstein was awarded the Nobel prize for Physics for his discovery about the photoelectric effect which explains how metals release electrons when exposed to light.
- Key Insight: The kinetic energy of emitted electrons depends on the frequency of light and not the intensity of light which disapproves the classical waving theory of light.
- Photon Concept: According to Einstein, the light consists of particles called photons and each carrying energy proportional to its frequency. When this energy exceeds a certain threshold limit it ‘shoots out an electron’.
- Solar Power Applications: This effect is especially important in the development of solar cells in which sunlight and its energy causes electron ejection and an ultimately electric current generation.
- Subatomic Probing: Photoelectric effect serves to uncover electronic properties, to investigate subatomic structures that contribute to its significance in modern-physics.
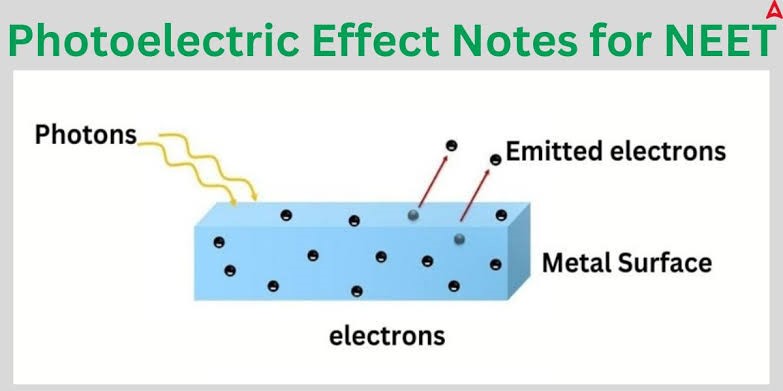
Understanding Photoelectric Effect
- Photoelectric effect is a phenomenon in which electrons are emitted out from the metallic surface when light is incident on it. These ejected electrons are referred to as photoelectrons.
- The emission of these photoelectrons and the kinetic energy of the emitted photoelectrons is dictated by the frequency of the light that is incident on the metal.
- The process that enables ejection of photoelectrons from the surface of metal due to action of light is well known as photoemission.
-
Photoelectric effect takes place due to the fact that electrons at the surface of the metal take energy from the light and apply it to overcome the forces which hold them in the surface of the metallic sphere.
Ultrafast Light Pulses and Their Role:
- Short Pulse Development: Ultrafast light pulses can be considered as an essential tool for studying the photoelectric effect, whose development became the basis for the 2022 Nobel Prize.
- Camera Analogy: At the atomic and molecular level, one might use shorter pulses of light which is similar to using the faster exposure time in camera and getting a sharper picture.
- Femtosecond Pulses: Femtosecond (10⁻¹⁵ seconds) pulses have been used to study atomic nuclei, enabling the observation of very short-lived atomic events.
- Attosecond Pulses: Researchers have developed attosecond pulses (10⁻¹⁸ seconds), allowing them to study faster-moving electrons and other quantum phenomena.
- Electron Dynamics: These pulses offer new insights into how electrons engage during a biochemical process and further enable the chance to dissect the complexities of electrons.
Delays in Photoionisation
- Study of Delays: Attosecond pulses reveal time delays between the moment a photon impacts an atom and the instant an electron is expelled, which gives essential data on molecular electronic configurations.
- Electronic Structure Mapping: Such delays assist physicists to establish the electronic potentials that determine molecular conduct in a way that could be useful in molecular design.
- 2010 Discovery: Work by other scientists as witnessed from the experiment revealed a 20 attosecond delay in the exit of the electron from two energy levels of a neon atom rather than simultaneously as expected.
- Nuclear Motion Role: New studies challenge the view that nuclear motion is too slow to influence electrons, where the present study focused on demonstrating that nuclear motion also causes fluctuations in photo-ionization delays.
- New Findings (2023): SLAC physicists also found that the process of photoemission of electrons from oxygen and nitrogen atoms takes place with a certain time lag, which expands knowledge on molecular processes.
Photomission Delay and Molecular Barriers
- Core Electron Delays: The SLAC researchers determined photoemission delays for core electrons in the X-ray regime that are much larger than observed before in ultraviolet experiments.
- Shape Resonance: It has been explained that electrons can be localized and delayed in a “shape resonance” for which the electron energy corresponds to the trapping potential.
- Barrier Interaction: Sometimes electrons require extra energy to overcome the barriers of molecules or it can pass through the molecular barriers because of quantum mechanical effects and hence delays.
- Auger-Meitner Effect: Another factor responsible for the delay is associated with the Auger-Meitner effect whereby an electron comes to fill a space left by a removed core electrons there being further electron delays.
- Molecular Sensitivity: Photoemission delays depend on the molecular structure and because of these interactions the nitric oxide molecules show larger delay than the nitrous oxide delays.
Expanding Applications of Attosecond Physics:
- X-ray Matter Interactions: The research performed at SLAC enriches the knowledge of X-ray interactions with matter which can be used for imaging proteins and viruses.
- Electron Correlation: Electron correlation which gives understanding of how electrons interact within molecules is critical for both fundamental science and real world applications like next-gen electronics.
- Biochemical Reactions: A deeper understanding of the behaviour of electrons contributes to improved notions on biochemical processes, and refinement of approach to formation of drugs and materials.
- Technological Advancements: These findings are therefore believed to help in the development of new materials to be used in the next generation electronics as well as better solar cells.
- Blue-Sky Research: Many basic findings on electrons’ behaviour prove to have new uses, which makes plain that fundamental research should continue.
Conclusion:
Further investigations of the phenomenon by using ultra-short light pulses are also shedding light on electron dynamics, materials properties, and molecular architecture. These findings may pave the way to upcoming innovations in solar energy, medical imaging, electronics, aspiring from the elastic potential of attosecond physics.
Source: The Hindu
Mains Practice Question:
Explain the importance of studying photoelectric effect in contemporary scientific production. In what way has an ultrafast light pulse enhanced the understanding of molecular structures and electronic behaviours? Explain with recent developments. (250 words)