SICKLE CELL DISEASE
Why in the News?
- The U.S. Food and Drug Administration (FDA) granted approval for two gene therapies targeting sickle cell disease.
- Lyfgenia, developed by bluebird bio, and Casgevy, a collaboration between Pharmaceuticals Vertex and CRISPR Therapeutics, received regulatory clearance.
Source: GBD
Utilization of CRISPR Gene Editing Technology
- Casgevy marks a significant milestone as the first-ever treatment for sickle cell disease utilizing the groundbreaking CRISPR gene editing technology.
- Both therapies are specifically sanctioned for individuals aged 12 years and older.
About CRISPR technology:
- Overview: CRISPR, or Clustered Regularly Interspaced Short Palindromic Repeats, is a gene editing technology inspired by bacteria’s natural defence mechanism against viruses, utilizing the Cas9 protein.
- Genetic Engineering Process: CRISPR involves manipulating genes, either adding new ones or suppressing existing ones, constituting a form of genetic engineering.
- No External Gene Introduction: Unlike some genetic engineering methods, CRISPR doesn’t introduce external genes but works with the organism’s existing genetic material through the CRISPR-Cas9 system.
- CRISPR-Cas9 as ‘Genetic Scissors’: CRISPR-Cas9 is often referred to as ‘Genetic Scissors’ for its precision in cutting and modifying genetic material, making it a powerful tool in genetic manipulation.
About Sickle Cell Disease
- It is an inherited blood disorder causing pain and a heightened risk of premature death.
- In sickle cell disease, red blood cells produce flawed, sickle-shaped haemoglobin, impeding the proper transport of oxygen and leading to complications such as intense pain, strokes, and organ failure.
Impact :
- Sickle cell disease, affecting around 100,000 individuals in the U.S., predominantly among the Black population.
Challenges and Considerations
- Despite being positioned as potential one-time treatments, uncertainties persist regarding the long-term efficacy of both gene therapies.
- Experts express caution, preferring to label these interventions as “transformative therapy” rather than outright cures due to continued challenges patients may face post-gene therapy.

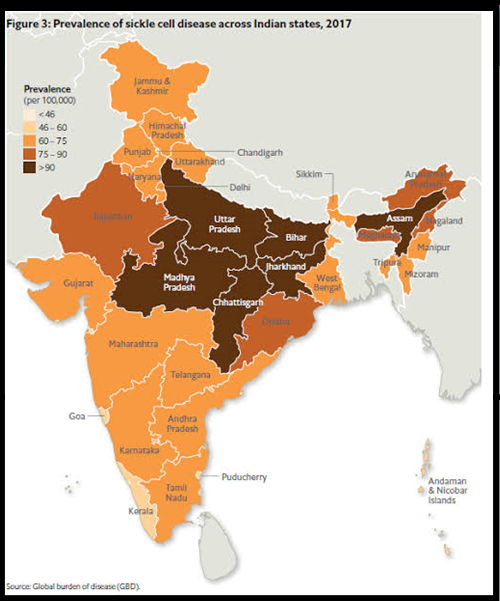 Source: GBD
Source: GBD

