SERIOUS ADVERSE EVENTS MAY OCCUR IN 1% OF COVAXIN RECIPIENTS
Why in the news?
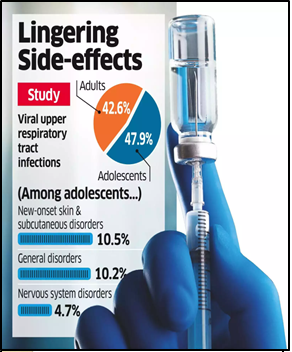
- A study published in Springer Nature highlights that adolescent girls and individuals with co-morbidities are at increased risk of adverse events after receiving Bharat Biotech’s Covexin (BBV152) vaccine.
- Nearly one-third of participants in the observational study reported adverse events of special interest (AESI).
- Conducted by Banaras Hindu University, the study titled “Long-term safety analysis of the BBV152 coronavirus vaccine in adolescents and adults” involved 1,024 participants.
source:toi
About Bharat Biotech:
- Bharat Biotech International Limited (BBIL) is an Indian multinational biotechnology company.
- Headquartered: in Hyderabad.
- Engaged in drug discovery, development, and manufacturing.
- Specialises in vaccines, biotherapeutics, pharmaceuticals, and healthcare products.
About (BBV152) Covaxin vaccine:
About Central Drugs Standard Control Organisation (CDSCO):
Associated Article: https://universalinstitutions.com/incovacc-intranasal-covid-19-vaccine/ |