Lithium – an overview
Relevance
- GS Paper 1 Distribution of Key Natural Resources, Mineral & Energy Resources.
- Tags: #Lithium #Lithiumionbattery #mining #REE #RareEarthElements.
Why in the news?
The Indian government has approved royalty rates for mining critical and strategic minerals, including Lithium, Niobium, and Rare Earth Elements (REEs). This move will enable the Central Government to auction mining blocks for these minerals for the first time. Royalty rates will be set at three percent of the London Metal Exchange price for Lithium, three percent of the Average Sale Price for Niobium, and one percent of the Average Sale Price of Rare Earth Oxide for REEs.
These actions align with India’s commitment to clean energy and achieving net-zero emissions by 2070. The recent Mines and Minerals (Development and Regulation) Amendment Act 2023 introduced an exploration license for deep-seated and critical minerals, facilitating private sector involvement in mineral exploration and delisting certain minerals, such as Lithium and Niobium, from the list of atomic minerals to allow concessions for private sector participation through auctions.
Key Points related to Lithium
Lithium is a soft, silvery-white metal that is the lightest of all solid elements. It is because of its strategic importance it is known as “White Gold”. It is a chemical element with the symbol Li and atomic number 3.
Physical Properties
- It is one of the least dense solid elements.
- It has a melting point of 180.5 degrees Celsius and a boiling point of 1,342 degrees Celsius.
- Lithium has a low density of 0.534 grams per cubic centimeter.
Chemical Properties
- Lithium is an alkali metal, and it is highly reactive. It readily reacts with water to form lithium hydroxide and hydrogen gas.
- Lithium is a part of the alkali metal group, which includes other elements such as sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr).
- These elements belong to Group 1 in the periodic table and are found in the s-block.
- It can also react with air and moisture to form a surface layer of lithium oxide and lithium hydroxide, which protects the underlying metal from further oxidation.
- Lithium is an excellent conductor of heat and electricity due to its high thermal and electrical conductivity.
- Abundance and Occurrence: Lithium is relatively rare in nature and is primarily found in the Earth’s crust, typically in the form of lithium-containing minerals such as spodumene, lepidolite, and petalite.
- It can also be extracted from certain lithium-rich brine deposits, often found in arid regions.
Rare Metal Classification
- Lithium is also categorized as a rare metal, which includes elements like niobium (Nb), tantalum (Ta), beryllium (Be), cesium (Cs), among others.
- These rare metals are strategically important due to their wide applications in high-tech industries, including electronics, telecommunications, information technology, space, defense, and nuclear technologies.
- In a broader context, rare metals are distinct from rare earth elements (REEs), which include elements from lanthanum (La) to lutetium (Lu), along with scandium (Sc) and yttrium (Y).
- Both rare metals and rare earth elements play crucial roles in advanced technologies and industries, with applications in various cutting-edge sectors.
Uses of Lithium
- Lithium-Ion Batteries: Widely used in portable electronics, electric vehicles (EVs), and renewable energy storage due to their high energy density and long cycle life.
- Pharmaceuticals: Lithium compounds like lithium carbonate are used to treat bipolar disorder and related mood disorders, helping stabilize moods.
- Nuclear Applications: Lithium is used in nuclear reactors to produce tritium and as a neutron source.
- Alloys: Lithium is alloyed with other metals to improve properties like strength and durability in aerospace and industrial applications.
- Greases and Lubricants: Lithium-based greases are used as lubricants in machinery and automotive applications.
- Air Treatment: Lithium bromide is used in air conditioning systems to absorb moisture from the air.
- Ceramics and Glass: Lithium compounds enhance the properties of ceramics and glass products.
- Primary Batteries: Lithium batteries (non-rechargeable) are used in devices like pacemakers, watches, and calculators.
- Aerospace and Defense: Lithium plays a role in aerospace technologies, including lightweight alloys and batteries for satellites and military applications.
- Electric Propulsion: In ion thrusters for space exploration, lithium is used as a propellant to generate thrust.
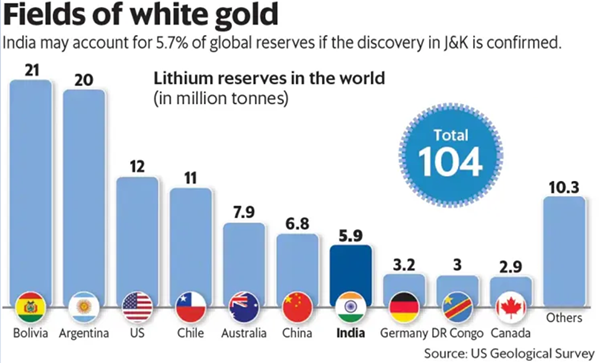
Lithium reserves Worldwide
Chile has the largest lithium reserves in the world, followed by Australia and Argentina. These three countries together account for over 50% of the world’s lithium reserves.
- As of 2023, the list of the top 10 countries with the largest lithium reserves: Chile > Australia > Argentina > United States > China > Bolivia > Canada > Brazil > Zimbabwe > Portugal
- Australia is the world’s largest producer of lithium, despite having the second-largest lithium reserves. This is because Australia has a well-developed mining industry and has invested heavily in lithium production
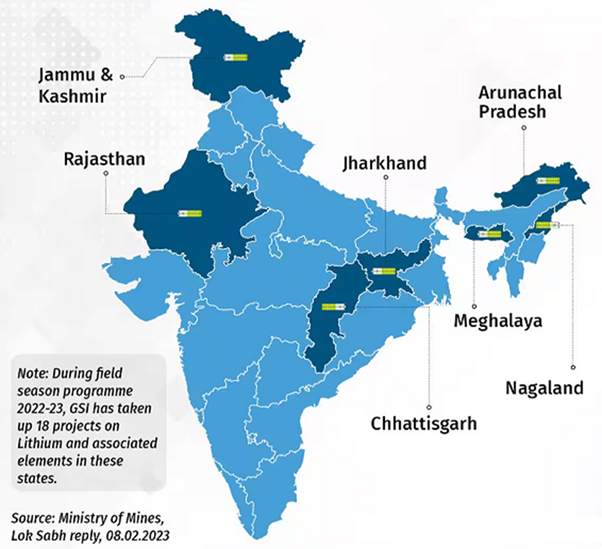
Lithium reserves in India
India has recently discovered significant lithium reserves in the states of Jammu and Kashmir, Rajasthan, and Jharkhand.
- The total estimated lithium reserves in India are estimated to be around 50 million tonnes, which is enough to meet the country’s demand for lithium for several decades.
- The largest lithium reserves in India are located in the Reasi district of Jammu and Kashmir.
- The Geological Survey of India (GSI) has estimated that the lithium reserves in this region are around 5.9 million tonnes.
- Another major reserve is located in the Nagaur district of Rajasthan with 40 million tonnes reserves.
Other Regions
- Brines of Sambhar and Pachpadra in Rajasthan, and Rann of Kachchh in Gujarat.
- The major mica belts in Rajasthan, Bihar, and Andhra Pradesh, and the pegmatite belts in Odisha and Chhattisgarh apart from Karnataka (Mandya Distt).
- In October 2023, The National Mineral Exploration Trust (NMET) reported the discovery of substantial reserves of numerous rare minerals, including lithium, in the regions of Koderma and Giridih in Jharkhand.
- The Indian government has announced that it will auction lithium mining rights to private companies.
- The government has also set up a National Lithium Corporation to oversee the development of the country’s lithium industry.
- The development of India’s lithium reserves is expected to help India to achieve its clean energy goals.
Why access and control over Lithium is important?
- Growing Electric Vehicle Market: India’s electric vehicle market has seen significant growth, and it’s projected to expand substantially, reaching a valuation of $152.21 billion by 2030.
- Import Dependency: India heavily relies on lithium imports, having imported 450 million units of lithium batteries worth $929.26 million (₹6,600 crore) in 2019-2020.
- Energy Transition: As the world shifts towards low-carbon economies to mitigate climate change, lithium’s role as a key component in renewable energy storage systems and clean transportation technologies has heightened its strategic importance.
- Technological Advancements: The rapid expansion of technologies like artificial intelligence (AI) and 5G networks demands high-capacity, long-lasting batteries for devices and infrastructure.
- Geopolitical Considerations: The geopolitics of lithium supply can influence a nation’s strategic positioning. Countries with access to lithium resources or reliable supply chains have a competitive edge in the global shift towards clean energy technologies, strengthening their diplomatic and economic influence.
- National Security: Dependence on lithium imports raises concerns about national security, as a disruption in the supply chain could impact the functioning of various critical sectors, including transportation, defense, and communication.
- Economic Opportunities: Developing domestic lithium extraction capabilities not only enhances energy security but also offers economic opportunities in terms of job creation, revenue generation, and reducing trade deficits.
- Innovation and Research: Control over lithium resources can spur innovation and research in battery technology, fostering advancements that go beyond EVs and touch various aspects of modern life.
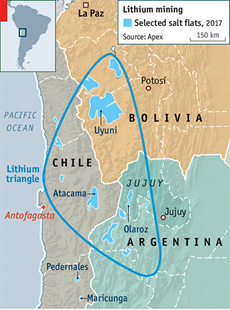
Lessons from South America
In the “lithium triangle” countries of Bolivia, Chile, and Argentina, which collectively hold approximately half of the world’s known lithium reserves, several noteworthy trends and considerations have emerged:
- Government Control: Bolivia and Chile have traditionally maintained state control over lithium extraction, often through agreements with state-owned entities, ensuring government authority over this valuable and strategically crucial resource.
- Environmental Responsibility and Community Engagement: In 2022, Chile revised compliance plan which underscores the significance of collaborative efforts among the company, regulatory bodies, and local communities to address environmental concerns, highlighting the importance of responsible resource extraction and community involvement.
- State Ownership and Restriction: Mexico introduced reforms in 2022 to establish a state-owned entity responsible for lithium extraction, processing, and sales. These reforms concurrently sought to limit private investment and production in the lithium sector, aligning with a broader regional trend of enhancing state control over lithium resources.
- Sustainable Resource Management: South American countries have confronted the challenge of harmonizing the economic advantages of lithium mining with environmental sustainability and social responsibility.
- Geopolitical and Economic Significance: The control of lithium resources in these nations carries substantial geopolitical and economic implications. It can significantly impact a country’s capacity to shape its energy landscape, advance its technological sector, and bolster its position in the global arena.
Safeguards for Indian mining sector
- Involvement of Local Communities: State officials in Jammu and Kashmir have emphasized the inclusion of local communities in lithium exploration projects, with a focus on offering job opportunities to these communities in exploration and mine development.
- District Mineral Foundation (DMF): The Mines and Minerals (Development and Regulation) Act of 1957 was amended in 2015 to establish the District Mineral Foundation (DMF). These foundations are non-profit statutory trusts created for each Indian district affected by mining operations, with the aim of benefiting people and areas affected by mining activities.
- Challenges in DMF Implementation: In practice, DMFs have faced challenges, as they have often become centralized bureaucracies with limited public participation or accountability. This situation highlights the need to ensure that these foundations effectively serve the interests of the affected communities and areas.
|
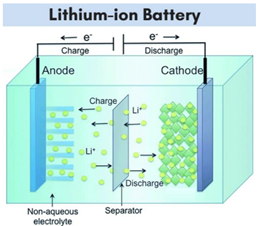
Lithium Ion Batteries A lithium-ion battery consists of four primary components:
Lithium-ion battery cells are assembled by arranging layers of electrodes and incorporating a porous membrane separator to isolate the anode and cathode. This separator facilitates the passage of ionic charge carriers while ensuring there is no electrical connection between the electrodes.
|
Source: Business today, Business Standard, TOI, Business Line
Mains Question
In the context of India’s growing focus on clean energy and technology, analyze the strategic significance and challenges of securing a sustainable supply of lithium metal for the nation’s development.