INDIGENOUS HPV VACCINE, THE RHETORIC AND THE REALITY
Syllabus:
GS 2:
- Issues Relating to Development and Management of Social Sector/Services relating to Health
GS 3:
- Achievements of Indians in Science & Technology; Indigenization of Technology.
Why in the News?
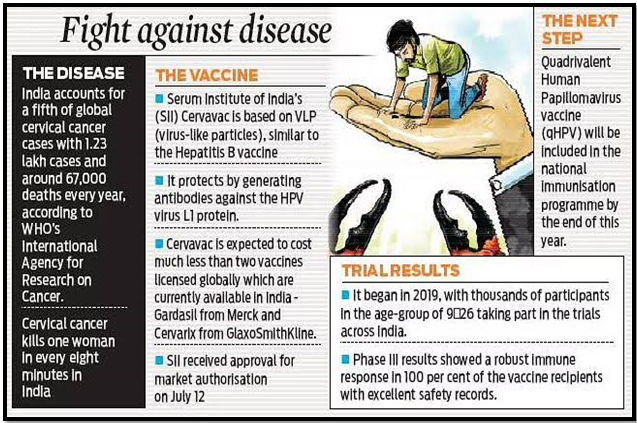
The recent launch and promotion of the indigenous HPV vaccine Cervavac have sparked debates on its timing, pricing, and the necessity of universal vaccination in India. This discourse highlights broader issues related to vaccine development, accessibility, and public health strategies in the country.
Source: IE
HPV Vaccine Discourse
- Public Debate: Recent discussions emphasize the role of HPV vaccination in preventing cervical cancer and deaths.
- Unproven Link: HPV’s direct causation of cervical cancer is not definitively proven; only a few strains are associated with precancerous lesions.
- HPV Prevalence: Most women who die of cervical cancer are HPV positive, but many HPV positive individuals do not develop cancer.
- Cancer Trends: Declining cervical cancer trends have been observed globally, regardless of vaccine coverage or efficacy.
- Vaccination Push: Overemphasis on universal vaccination may overshadow the need for selective vaccination of high-risk groups.
Human Papillomavirus (HPV)
Transmission
HPV Vaccination
|
Western Assumptions
- Risk Assumptions: Targeting pre-puberty girls assumes teenage promiscuity as a major risk factor, which may not align with Indian societal norms.
- Moral Conundrum: The approach raises moral questions and can be seen as patriarchal, as men can also be carriers of the virus.
- Selective Vaccination: Considering HPV’s sexual transmission, selective vaccination of high-risk groups may be more justified.
- Public Concerns: There is significant public concern about the appropriateness and implications of the vaccination strategy.
- Cultural Context: The vaccination strategy needs to be evaluated within the cultural and social context of Indian society.
Vaccine Development Path
- Questionable Timing: The timing, promotion, and pricing of indigenous HPV vaccines like Cervavac are under scrutiny.
- Development Delay: It took nearly two decades for an indigenous HPV vaccine to be developed after patented versions in the Global North.
- Production Techniques: Cervavac uses virus-like particles produced with recombinant DNA techniques, similar to earlier vaccines.
- Historical Shift: Vaccine manufacturing has shifted from a charitable/public sector model to a patent-driven private sector model since the 1980s.
- Innovation Changes: The U.S. patent law changes and globalisation led to significant shifts in vaccine innovation, patenting, and distribution practices.
Impact on India
- Patent Delays: Indian vaccine development was delayed due to global patent regimes, affecting the availability of affordable vaccines.
- Price Concerns: The high current market price of Cervavac remains unexplained, despite local production capabilities.
- Funding Influence: Significant funding from sources like the Gates Foundation and shared infrastructure should lower production costs.
- Affordability Issue: The pricing strategy of Cervavac appears to prioritize high margins over affordability and public health needs.
- Public Health: High population coverage is essential for vaccine success, which requires affordable pricing and wide accessibility.
Lack of Competition
- Limited Alternatives: The lack of competing HPV vaccines from other domestic players has limited downward pressure on prices.
- Pipeline Projects: Several domestic vaccine candidates have been in development since 2010 but are still unavailable in the market.
- Acquisition Impact: The acquisition of domestic players by multinational companies has impacted the availability of affordable vaccines.
- Government Pricing: The current government-recommended price for Cervavac is still considered expensive, even at a subsidized rate.
- Public Interest: The lack of competition and opaque pricing strategies merit investigation to ensure public health interests are safeguarded.
Way Forward
- Strengthen Research: Invest in research to better understand the link between HPV and cervical cancer, ensuring vaccination strategies are based on robust scientific evidence.
- Selective Vaccination: Prioritize selective vaccination for high-risk groups rather than universal vaccination, optimizing resource allocation and addressing the most vulnerable populations.
- Improve Accessibility: Implement pricing strategies that make HPV vaccines affordable for all segments of the population, particularly the economically disadvantaged.
- Promote Competition: Encourage domestic vaccine manufacturers to bring their HPV vaccines to market, increasing competition and driving down prices.
- Transparent Pricing: Ensure transparent pricing mechanisms that reflect actual production costs and funding sources, promoting fairness and accessibility.
- Public Awareness: Increase public awareness about HPV, its transmission, and the benefits and limitations of vaccination, addressing moral and cultural concerns.
- Policy Support: Develop supportive policies that facilitate the swift introduction of affordable and effective vaccines while maintaining high standards of safety and efficacy.
- Global Collaboration: Foster international collaboration to share research findings, production techniques, and best practices, enhancing global efforts in HPV prevention and cervical cancer reduction
Conclusion
Ensuring equitable access to affordable and effective HPV vaccines while addressing scientific, ethical, and economic concerns is crucial for public health. A balanced approach focusing on selective vaccination and competitive pricing can better serve India’s diverse population and enhance cervical cancer prevention efforts.
Source:The Hindu
Mains Practice Question:
Discuss the challenges and opportunities associated with the introduction of the indigenous HPV vaccine Cervavac in India. How can public health policy be optimized to address these issues?
Associated Article: