India’s Biobanks And Precision Medicine: Gaps And Growth
Syllabus:
GS-3:
Biotechnology ,Scientific Innovations & Discoveries
Focus:
India’s precision medicine sector is rapidly growing, but inadequate biobank regulations are hindering its full potential. With biobanks playing a crucial role in medical research, the need for strong data protection laws and ethical oversight has come to the forefront, pushing for reforms.
Introduction to Precision Medicine and Biobanks:
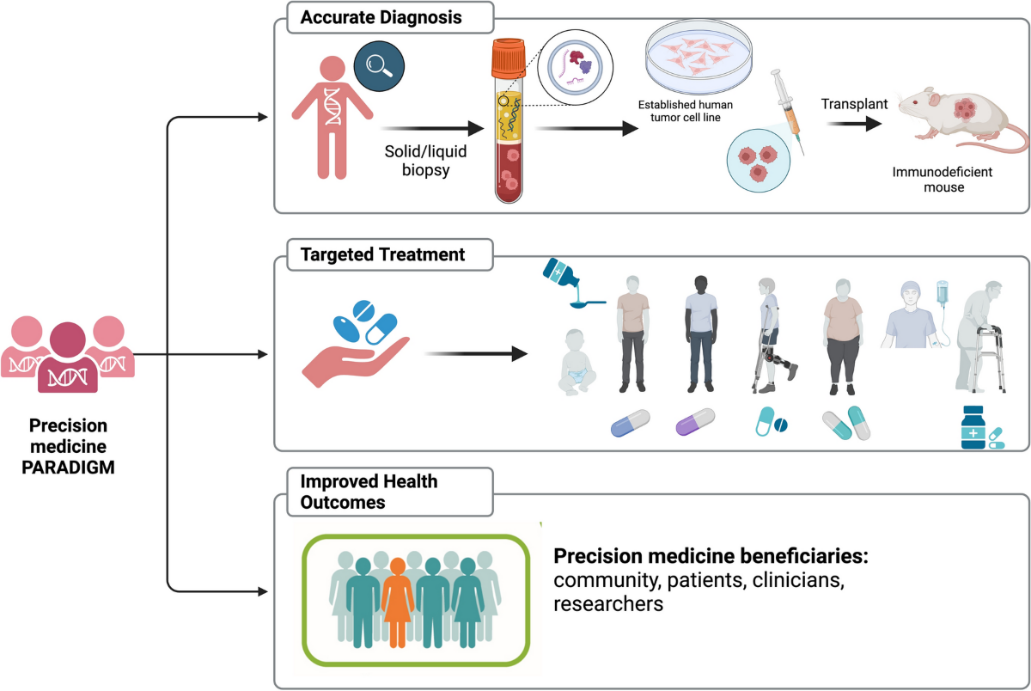
- Precision Medicine Overview: Precision medicine is advancing personalised healthcare, greatly aided by genomics, gene editing, and mRNA technologies.
- Historical Milestones: It gained momentum with the Human Genome Project and has since been used to treat cancers, chronic, immunological, cardiovascular, and liver diseases.
- Recent Successes: Notable examples include using gene therapy to restore vision and reversing diabetes through reengineered stem cells in the U.K.
- Technological Contributions: mRNA vaccines, which emerged during COVID-19, exemplify rapid precision medicine solutions, winning a Nobel Prize for the technology.
- Organ-on-Chips: These microfluidic devices replicate human organs and promise advancements in drug testing and precision medical solutions.
What is Precision Medicine and Biobanks?
- Precision Medicine: Customises medical treatment based on individual genetic makeup, lifestyle, and environmental factors.
- Biobanks: Repositories storing biological samples (blood, tissues, DNA) with genetic data from consenting individuals for research.
Importance of Biobanks in Precision Medicine:
- Data Repository: Provides essential genetic data for identifying disease patterns.
- Research Backbone: Facilitates studies on genetic disorders and personalised treatments.
- Diversity in Research: Large, diverse biobanks enhance the applicability of precision medicine across populations.
Present Status in India:
- Growth of Precision Medicine: Expected market worth over $5 billion by 2030.
- Biobanks: 19 registered biobanks; initiatives like Genome India and Phenome India are underway.
- Policy Gaps: Lack of comprehensive biobank regulations hinders growth.
Best Practices from Foreign Nations:
- Comprehensive Laws: Countries like the U.S., U.K., Japan, and China regulate biobanks covering consent, privacy, and data security.
- Oversight: Centralised authorities ensure ethical standards and data protection.
Biotechnology Applications in Medicine:
- Drug Development:
- Biopharmaceuticals: Producing drugs like proteins and vaccines from biological sources.
- Pharmacogenomics: Personalising medicine based on genetic differences.
- High-throughput screening: Rapid drug candidate testing.
- Diagnostics:
- Molecular diagnostics: PCR and sequencing for disease detection.
- Companion diagnostics: Identifying biomarkers for targeted therapies.
- Gene and Cell Therapies:
- Gene therapy: Inserting healthy genes to correct genetic disorders.
- CRISPR: Precise gene editing.
- Regenerative Medicine:
- Tissue engineering: Creating organs and tissues using biomaterials and stem cells.
- Medical Devices:
- Biosensors and lab-on-a-chip for rapid diagnostics and disease monitoring.
About Mission on Paediatric Rare Genetic Disorders (PraGeD):
- Initiative Overview: A PAN-India program funded by the Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India.
- Collaboration: Works with pediatric departments of medical colleges and DBT’s diagnostic centres under the UMMID program.
- Objective: Analyse samples from children with rare genetic disorders and their parents.
- Facilities: The Centre for DNA Fingerprinting and Diagnostics (CDFD) is developing advanced facilities for studying pediatric rare genetic diseases.
- Research Focus: High-throughput Whole Exome Sequencing (WES) and Whole Genome Sequencing (WGS) to identify gene mutations causing rare diseases.
- Data Management: Create a phenotype-genotype database to determine the genetic basis of these conditions.
Precision Medicine in India:
- Growth of the Sector: India’s precision medicine market is projected to grow at a CAGR of 16%, reaching over $5 billion by 2030.
- Bioeconomy Contributions: Precision medicine, alongside cancer immunotherapy, gene editing, and biologics, forms 36% of India’s bioeconomy.
- Government Initiatives: India’s first domestically developed CAR-T cell therapy (NexCAR19) was approved in 2023, showcasing a strong governmental focus.
- Infrastructure Expansion: New centres, such as those at Apollo Cancer Centre and a collaboration between Siemens Healthineers and IISc Bengaluru, are focused on AI for precision medicine.
- BioE3 Policy: The new BioE3 policy includes development strategies for precision therapeutics, signalling a long-term focus on this field.
Role of Biobanks in Precision Medicine:
- Definition and Importance: Biobanks store biological samples like DNA, tissues, and organs, crucial for research in precision medicine.
- Need for Diversity: To benefit the entire population, biobanks must be large and diverse. Otherwise, only certain segments of society will benefit from medical research.
- Recent Studies: A biobank was used to identify people with rare genetic disorders, while another study created a large sarcoma organoid biobank to identify therapies.
- Advantages for Research: Biobanks allow researchers to test drugs and develop therapies by creating miniaturised organ systems for high-throughput drug screening.
- Ethical Challenges: Biobanks can also raise ethical issues if not properly regulated, including concerns over data use, consent, and potential exploitation.
Biobanks and Regulations in India:
- Current Biobank Landscape: India has 19 registered biobanks, collecting various biological specimens, including those for rare genetic diseases.
- Genome and Phenome Projects: The Genome India program sequenced 10,000 genomes, and the Phenome India project collected 10,000 samples to improve disease prediction models.
- Pediatric Mission: The Paediatric Rare Genetic Disorders (PRaGeD) mission focuses on genetic diseases in children, aiming to develop targeted therapies.
- Regulatory Gaps: India lacks comprehensive biobank regulations, leaving critical gaps in informed consent, data protection, and sample ownership rights.
- Global Comparison: Unlike countries like the U.S., U.K., and China, India has yet to implement robust biobank laws, which affects trust and participation.
Ethical Concerns in Biobanking:
- Informed Consent: Participants often provide samples without understanding how their data will be used or who will access it.
- Data Privacy: Genetic data can reveal sensitive information about individuals and their families, raising concerns about privacy breaches.
- Discrimination Risks: Genetic data misuse could lead to discrimination, especially in healthcare or insurance sectors.
- Lack of Oversight: Without a single regulatory authority, there is potential for sample mishandling and unethical sharing of data.
- Global Disparities: Countries with robust biobank regulations prevent such issues, making India’s regulatory gaps more prominent.
Benefits of Strengthening Biobank Regulations:
- Increased Participation: Stronger protections for sample donors will encourage wider public participation in biobanking.
- Global Collaboration: With clear regulations, Indian biobanks can collaborate more easily with international pharmaceutical companies.
- Enhanced Research: Proper oversight will lead to more accurate and ethically conducted research, benefiting precision medicine.
- Economic Growth: A well-regulated biobank system could boost India’s bioeconomy by attracting global research investments.
- Public Trust: Clear laws will enhance public trust in the medical system, fostering long-term support for precision medicine advancements.
Opportunities and Future Directions:
- Importance of Regulation: Stronger laws and ethical frameworks are needed to protect data privacy and ensure proper use of biobank samples in India.
- Public Trust: Ensuring transparency in how samples are used will encourage more individuals to contribute to biobanks without fear of misuse.
- Pharmaceutical Collaboration: Properly regulated biobanks will attract pharmaceutical companies for research, fostering collaboration without exploitation.
- Global Leadership: India, as part of international groups like BRICS and the Quad, has a chance to lead in precision medicine by aligning biobanking laws with global standards.
- Conclusion: If India strengthens its regulations, it can expand its leadership in next-generation therapeutics, reinforcing its position as a pharmaceutical hub.
Conclusion:
Biotechnology’s advancements in medicine, from drug development to gene editing and regenerative therapies, are revolutionising healthcare. By enabling personalised treatment and precise diagnostics, biotechnology holds immense potential to improve patient outcomes and address complex medical challenges, ultimately transforming the future of healthcare.
Source: The Hindu
Mains Practice Question:
Discuss the role of biotechnology in revolutionising modern medicine, focusing on its applications in diagnostics, drug development, and regenerative therapies. How can India leverage these advancements to enhance its healthcare system and improve patient outcomes? (250 words)