“ICMR DISAVOWS BHU STUDY ON COVAXIN, CITES CRITICAL FLAWS”
Why in the news?
- The ICMR disassociated from a BHU study on Covaxin, citing no financial or technical support and critical design flaws.
- ICMR criticized the study for lacking a control group and proper documentation of adverse events.
Source:IndiaToday
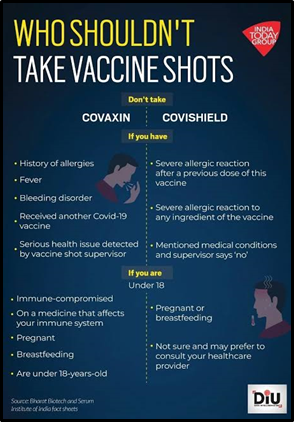
Key Points about COVAXIN:
- “COVAXIN is India’s first indigenous COVID-19 vaccine developed by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR).
- Type: Inactivated vaccine
- Development: Inactivates (kills) live microorganisms causing the disease
- Mechanism:Destroys pathogen’s ability to replicate
- Targets:Spike protein and Nucleocapsid protein (virus shell enclosing genetic material)
- Significance: Contains immunogens (epitopes) from other genes in addition to Spike protein
About Indian Council of Medical Research (ICMR):
About Banaras Hindu University (BHU):
Associated Article: https://universalinstitutions.com/serious-adverse-events-may-occur-in-1-of-covaxin-recipients/ |