Gene Therapy in 2025: Ushering in a New Era of Curative Medicine
Gene Therapy in 2025: Ushering in a New Era of Curative Medicine
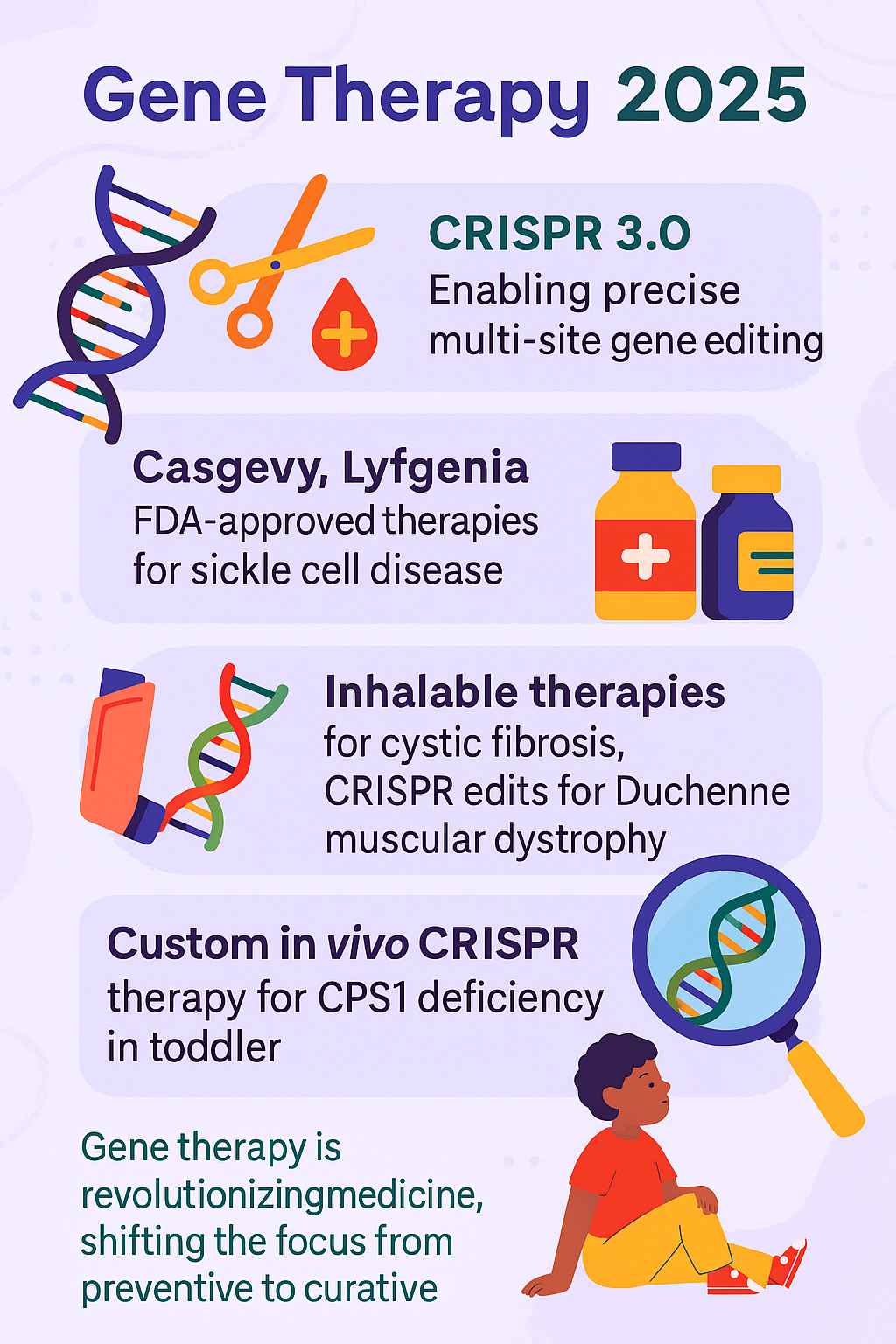
Genetic therapy has long stood at the frontier of biomedical science, but in 2025, it is no longer just a promise of the future—it is a transformative medical reality. With the advent of CRISPR 3.0, a more refined and versatile gene-editing system, medical researchers have taken significant strides toward curing diseases previously considered chronic or untreatable. From FDA-approved therapies to groundbreaking trials in paediatric medicine, cell and gene therapy is not only changing lives—it is fundamentally redefining the way we understand and approach treatment. The first gene therapy approved by the FDA marked a pivotal moment in medical history, paving the way for the remarkable advancements we see today.
The CRISPR 3.0: Precision at the Molecular Level
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technology has evolved significantly since its discovery. The latest iteration, CRISPR 3.0, introduces multi-locus and tissue-specific editing with improved off-target precision, allowing scientists to simultaneously edit multiple genes or genetic regions with high accuracy. It integrates next-generation guide RNAs and engineered nucleases that reduce the risk of collateral damage to non-target DNA, representing a significant advancement in genome editing techniques. This technology has greatly enhanced the safety and effectiveness of gene therapy treatments.
FDA Approvals: Casgevy and Lyfgenia for Sickle Cell Disease
In a landmark moment for genetic therapy, the U.S. Food and Drug Administration (FDA) approved Casgevy and Lyfgenia, two gene therapy examples for sickle cell disease (SCD)—a debilitating hereditary blood disorder affecting millions globally. These approvals mark a significant milestone in sickle cell disease treatment and potentially offer a sickle cell anemia cure.
- Casgevy (developed by Vertex Pharmaceuticals and CRISPR Therapeutics) uses ex vivo CRISPR-Cas9 gene editing to modify the BCL11A gene, boosting fetal hemoglobin production and effectively preventing sickling of red blood cells.
- Lyfgenia (developed by Bluebird Bio) employs a lentiviral vector to insert a functioning copy of the beta-globin gene into the patient’s hematopoietic stem cells, representing an innovative approach to cell-based gene therapy.
Both therapies mark a shift from lifelong symptomatic treatments to one-time interventions with curative potential. Early clinical results indicate that over 90% of treated patients remain symptom-free beyond 12 months post-treatment, with improved quality of life and reduced hospitalization. These advancements also show promise for addressing sickle cell trait treatment in the future and reducing vaso-occlusive events, a common complication of the disease.
Breakthroughs in Inhalable Gene Therapy for Cystic Fibrosis
Cell and gene therapy has shown immense promise in treating cystic fibrosis (CF), a chronic disease caused by genetic mutations in the CFTR gene. In 2025, researchers have successfully developed inhalable lipid nanoparticle-based CRISPR therapies that directly deliver gene-editing payloads to lung epithelial cells via aerosol sprays. This innovative approach complements other delivery methods, such as intraocular implants for ocular diseases, showcasing the versatility of gene therapy applications.
These cellular therapy approaches have:
- Corrected faulty CFTR gene expression in human lung cells
- Restored near-normal mucus clearance and lung function in animal and early-phase human trials
- Shown minimal inflammatory response, suggesting strong biocompatibility for chronic use
If phase III trials confirm efficacy, this could become the first non-invasive gene therapy for a systemic genetic disorder, offering a lifeline to thousands and expanding the cell and gene therapy market. The development of such stem cell products and cell product innovations is crucial for advancing the field.
Pediatric Gene Therapy: CPS1 Deficiency Successfully Treated
One of the most celebrated successes of 2025 is the use of custom in vivo cell therapy to treat a toddler diagnosed with Carbamoyl Phosphate Synthetase 1 (CPS1) deficiency, a rare and often fatal metabolic disorder. Using a viral vector tailored to the child’s unique genetic mutation, scientists:
- Delivered CRISPR components directly into the liver cells
- Achieved over 80% correction in hepatic enzyme activity
- Allowed the child to transition off ammonia-scavenging drugs within weeks
This case marks the first successful in vivo, patient-specific CRISPR treatment in a child under 3 years old, demonstrating the viability of personalized gene therapy for ultra-rare diseases. The treatment protocol included myeloablative conditioning to prepare the patient’s body for the gene therapy, a crucial step in ensuring its success.
Expanding Horizons: Upcoming Trials for Neurological and Metabolic Diseases
With encouraging results in haematological and metabolic disorders, cell therapy treatment is rapidly expanding into more complex domains, including:
- Neurodegenerative diseases like Huntington’s, ALS, and certain types of Parkinson’s
- Metabolic syndromes such as phenylketonuria and glycogen storage diseases
- Rare diseases like Batten disease and spinal muscular atrophy
- Hemophilia, which has shown promising results in early trials
Ongoing clinical trials are using advanced CRISPRi (CRISPR interference) and epigenetic editing techniques to modulate gene expression without altering DNA sequences—ideal for reversible therapies in the central nervous system. Additionally, RNA therapies and RNAi technologies are being explored for their potential in treating various genetic disorders. The pipeline overview for these emerging therapies is extensive, with RNAi therapy showing particular promise in targeting specific genetic mutations associated with rare diseases.
From Preventive to Curative: A Paradigm Shift in Medicine
Until now, much of modern medicine has operated within a preventive or palliative model—aiming to delay or manage symptoms. Gene cell therapy transforms this model into one that is fundamentally curative:
- No more daily medications or lifelong treatments
- One-time interventions with potentially lifelong effects
- Personalized to genetic profiles, not “one-size-fits-all”
This transition will not only improve patient outcomes but could drastically reduce long-term healthcare costs, ushering in a new age of precision medicine. The cell and gene therapy market is expected to grow significantly as more therapeutic products become available. Long-term studies are underway to monitor the durability and safety of these groundbreaking treatments, with a focus on understanding the long-term effects of gene therapy interventions.
Ethical and Accessibility Considerations
Despite its promise, genetic therapy raises ethical concerns:
- Who gets access first: high-income patients or those with the greatest need?
- What are the risks of off-target effects in somatic or germline editing?
- How do we ensure long-term monitoring and regulation?
- What are the implications of “CRISPR babies” and genetic modification in embryos?
Global regulatory frameworks and bioethics councils are now working in tandem with scientists to ensure responsible and equitable deployment of these therapies. Issues such as start-up funding, dealmaking, and priority review are being addressed to accelerate the development and accessibility of these groundbreaking treatments.
Conclusion: Medicine Reimagined
Gene therapy in 2025 is more than a medical advancement—it is a revolution. As CRISPR 3.0 and allied technologies make treatments faster, safer, and more precise, the world is witnessing the dawn of curative medicine. Whether it’s a toddler freed from metabolic disease, a young adult no longer burdened by sickle cell crises, or an elder breathing easier with gene-corrected lungs—this is science healing at the source.
As clinical trials expand and regulatory bodies adapt, genetic therapy is no longer a miracle in the making—it’s a medical movement in motion. The integration of cellular therapy, RNA therapies, and RNAi approaches is paving the way for innovative treatments for a wide range of rare diseases and genetic disorders. With ongoing research in areas such as mesenchymal stem cell therapy, base editing, and non-genetically modified cell therapies, the future of medicine looks brighter than ever. The continuous improvement in safety and effectiveness of these treatments promises a new era of curative medicine, transforming the lives of patients worldwide. Long-term studies will be crucial in assessing the enduring impact and safety profile of these revolutionary therapies, ensuring their sustained success in the years to come.