Each year a large amount of plant material, cellulose, is deposited on the surface of Planet Earth. What are the natural processes this cellulose undergoes before yielding carbon dioxide, water and other end products?
Introduction
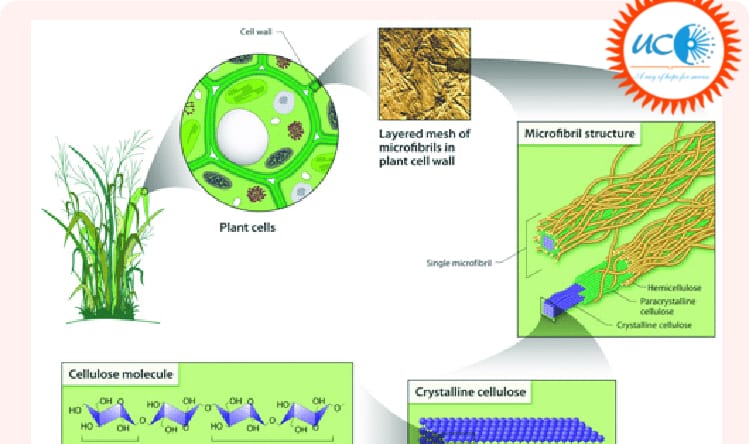
Cellulose is a structural component of plant cell walls. Cellulose is helping plants to remain stiff and upright. Because of its physical properties, it is important in determining the orientation of cell expansion.
Body
Natural processes that cellulose undergoes before yielding carbon dioxide, water and other end products.
- Cellulolysis is a hydrolysis reaction that converts cellulose into cellodextrins, which are smaller polysaccharides, or entirely into glucose units. When compared to the breakdown of other polysaccharides, cellulolysis is relatively challenging due to the strong molecular bonds that cellulose molecules have with one another.
- Cellulose undergoes thermolysis, sometimes known as “pyrolysis,” at temperatures exceeding 350 °C, breaking down into solid char, vapours, aerosols, and gases like carbon dioxide. At 500 °C, the highest production of vapours that condense into bio-oil is produced. It has been demonstrated that semi-crystalline cellulose polymers react at pyrolysis temperatures (350–600 °C) in a matter of seconds, with the liquid (referred to as intermediate liquid cellulose or molten cellulose) remaining for only a brief period of time.
- The melt is made up of two to seven monomer long short cellulose chains as a result of glycosidic bond breaking. Aerosols are created by the vapour bubbling of intermediate liquid cellulose and contain short-chain anhydrous-oligomers that are extracted from the melt.
- Levoglucosan, furans, pyrans, light oxygenates, and gases are among the volatile substances that are produced while molten cellulose continues to decompose through primary reactions. Levoglucosan and other volatile substances conduct “secondary reactions” with pyrans and light oxygenates, such as glycolaldehyde, within samples of thick cellulose.
Conclusion
Cellulose degradation is a prime example which undergoes both organic and inorganic decomposition to maintain the balance in nature.