DRUG REGULATOR WARNS ABOUT MEROPENEM, DISODIUM
Why in the news?
CDSCO warns against unapproved drugs like Meropenem and Disodium EDTA, stressing the need for proper approvals before manufacture and sale.
source:slideshare
About Warning Against Unapproved Drugs:
- The Central Drugs Standard Control Organisation (CDSCO) issued a caution against the manufacture and sale of unapproved drugs, particularly emphasising “New Drugs.”
- Mentioned specific examples like Meropenem (antibacterial agent) and Disodium EDTA (used for treating calcium overload).
- Noted instances of manufacturers engaging in the production or marketing of drugs not yet approved by the CDSCO.
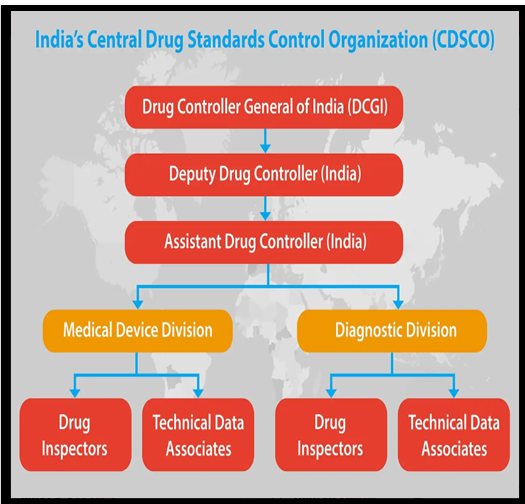
| About The Central Drugs Standard Control Organisation (CDSCO) :
● CDSCO is India’s National Regulatory Authority for the medical devices industry. ● It operates under the Ministry of Health & Family Welfare. ● The Drugs Controller General of India (DCGI) heads the CDSCO. ● Headquarters: New Delhi. ● Responsibilities include approving new drugs, conducting clinical trials, setting drug standards, and ensuring imported drug quality. ● CDSCO grants licences for critical drugs such as blood products, IV fluids, vaccines, and sera. |