ALARMING PRESENCE OF UNAPPROVED ANTIBIOTIC COMBINATIONS
Why in the News?
- Academics from India, Qatar, and the UK reveal a concerning study on unapproved and banned Fixed-Dose Combinations (FDCs) of antibiotics in India.
Key Highlights of the Study:
- Data : 5% of antibiotic FDCs (239 formulations) were unapproved(Year 2020).
Additional 9.9% (39 formulations) were banned but still sold in the country.
- Impact: Unapproved or banned FDCs containing antibiotics raise concerns about increasing Antimicrobial resistance(AMR) in India.
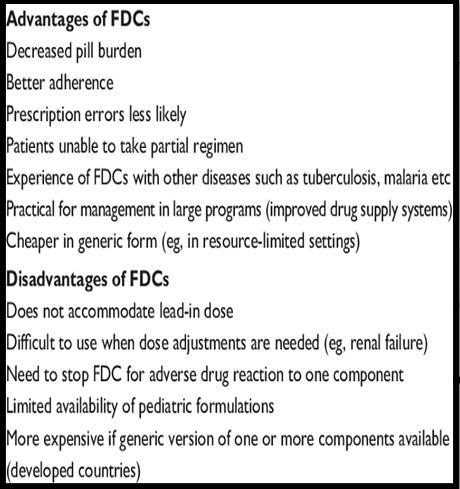
- Significance of FDCs: FDCs combine known drugs for improved patient compliance.
For Example: AIDS treatment benefits from FDCs, enhancing patient adherence and treatment outcomes.
Challenges in FDC Development:
- Despite safety and efficacy profiles, formulating FDCs is complex.
- Potential interactions between active ingredients or excipients may impact therapeutic efficacy or create toxic elements.
Source: Research Gate
Pharmaceutical Industry’s Exploitation of FDCs
- Avoiding Regulation:
- Companies in India exploit FDCs to evade liability under laws like the Drugs (Prices Control) Order (DPCO).
- FDCs provide a loophole as drug combinations were traditionally not covered under DPCO.
- Proliferation of Unjustified FDCs:
Industry introduces FDCs without medical rationale.
For Examples: Combining an anti-inflammatory drugs with vitamins, contributing to a bewildering variety.
- Advantages for Manufacturers:
- Lack of standards for FDC testing due to the diverse market offerings.
- FDCs allow companies to charge higher prices by claiming uniqueness and catering to specific needs.
Regulatory Challenges and History
Regulatory Framework Ineffectiveness (1978-Present):
Initial lack of a system to vet drugs for safety and efficacy under the colonial-era Drugs and Cosmetics Act, 1940.
Attempts to Address Issues (1982-1988):
- Amendments in 1982 grant central government power to prohibit drugs lacking therapeutic value.
- 1988 amendments mandate safety and efficacy proof submission for all new drugs, including FDCs.
Reasons for regulatory Failure:
- Disregard for Regulatory Measures: State drug controllers continue issuing manufacturing licenses for unapproved FDCs, violating established laws.
- Ineffectiveness of Section 26A Powers:
Lack of criminal prosecutions against manufacturers selling unapproved FDCs under Section 26A to prohibit specific FDCs
Unregulated FDCs may contribute to the AMR problem, emphasizing the need for immediate Ministry of Health action.

 Source: Research Gate
Source: Research Gate

