Why Pencils Write: The Science of Graphite
Why in the news?
A detailed explanation of why pencils can write, focusing on graphite’s unique atomic structure, highlights the interplay of carbon atoms and condensed matter physics in everyday materials.
Composition of a Pencil Core:
- The core of a pencil is made of graphite, a form of carbon.
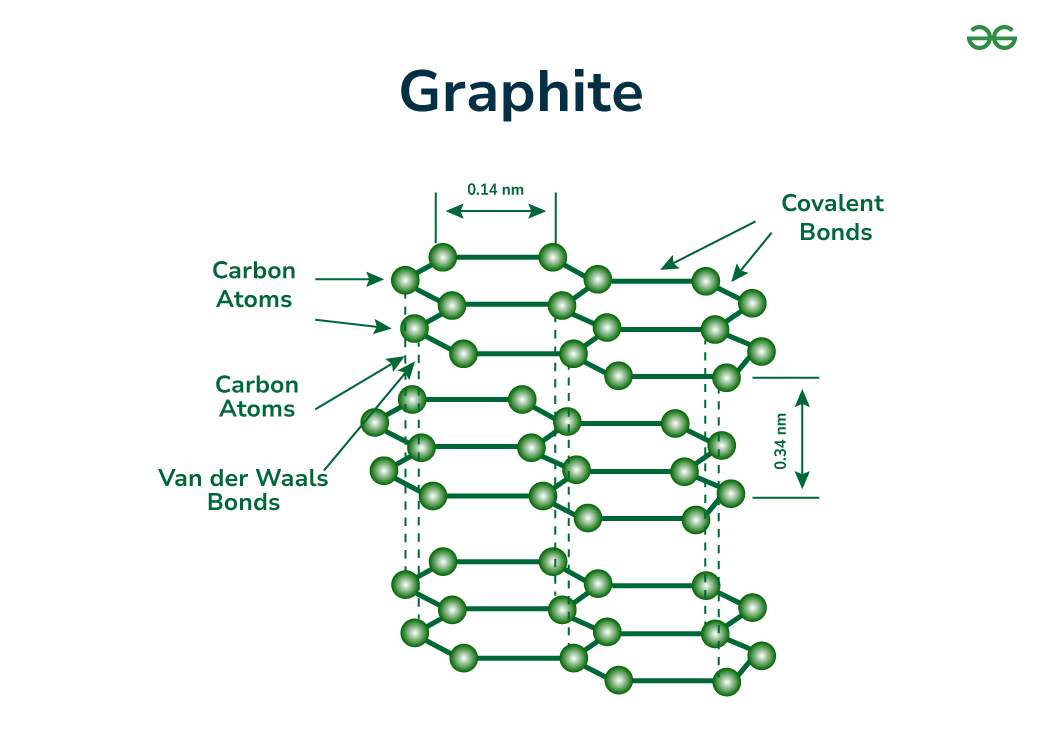
- Unlike diamonds, where carbon atoms are tightly bonded in a rigid structure, graphite’s carbon atoms are arranged in layers, called graphene.
- These layers are weakly bonded to each other, allowing them to slide off easily.
Mechanism of Writing:
- Writing occurs as layers of graphite transfer from the pencil onto the paper.
- The act of pressing the pencil on paper forces the upper layers of graphene to slide off and adhere to the surface.
- This sliding mechanism is what differentiates graphite from materials like diamonds, whose tightly bound structure prevents them from transferring atoms.
The Science Behind the Process:
- The unique arrangement of carbon atoms in graphite makes it black and soft, ideal for leaving visible marks.
- This phenomenon is rooted in condensed matter physics, which studies how the arrangement of atoms affects material properties.
- Graphite’s behaviour serves as an example of how different atomic structures lead to varied material characteristics, even when composed of the same element.
What is Graphite?
- About:
- Graphite is a naturally occurring carbon-based mineral.
- It is one of the three crystalline forms of carbon, alongside diamond and amorphous carbon (e.g., charcoal).
- Structure:
- Composed of carbon atoms arranged in hexagonal sheets.
- Layers are weakly bonded, enabling them to slide past one another.
- Properties:
- Excellent conductor of electricity and heat.
- Offers lubricating properties due to its sliding layers.
- Applications:
- Used in pencils (graphite and clay mix).
- Employed in batteries, electric motor brushes, crucibles, and nuclear reactor cores.
- Global Reserves:
- Turkey and Brazil hold 50% of global natural graphite resources.
- China ranks third with 16%, followed by Madagascar (7.9%).
Sources Referred:
PIB, The Hindu, Indian Express, Hindustan Times