HOW DANGEROUS IS METHANOL POISONING?
Syllabus:
GS 2: Issues Relating to Development and Management of Social Sector/Services relating to Health
GS 3: Disaster and disaster management.
Why in the News?
- At least 38 people had died after consuming spurious liquor in the Kallakurichi district of Tamil Nadu while as many as 82 others were receiving treatment in hospitals.;
About Methanol
|
How Dangerous is Methanol Poisoning?
Toxicity and Lethal Effects
- Minimal Lethal Dose: More than 0.1 ml of pure methanol per kilogram of body weight can be devastating.
- Metabolic Acidosis: Methanol is metabolized to formic acid, which leads to metabolic acidosis, causing the blood to become increasingly acidic.
- Optic Neuropathy: Methanol consumption can cause methanol-induced optic neuropathy, potentially leading to long-term or irreversible visual impairment or blindness.
- Cerebral Edema and Hemorrhage: Methanol poisoning can cause cerebral edema and hemorrhage, which are often fatal.
- Systemic Toxicity: Methanol disrupts cellular respiration by interfering with cytochrome oxidase, leading to a buildup of lactic acid and further contributing to acidosis.
Effects on the Body
- Formaldehyde Formation: Methanol is metabolized to formaldehyde in the liver, a highly toxic substance.
- Formic Acid Accumulation: Formaldehyde is further metabolized to formic acid, which accumulates and leads to systemic toxicity.
- Disruption of Cellular Respiration: Formic acid interferes with cellular respiration, causing cells to be unable to use oxygen effectively.
- Acidosis: The accumulation of formic acid leads to a dangerous drop in blood pH levels.
- Visual Impairment: Damage to the optic nerve and retina from formic acid can lead to permanent visual impairment or blindness.
Incident in Kallakurichi District, Tamil Nadu
Tragic Event
- Death Toll: As of June 20, at least 38 people had died after consuming spurious liquor in the Kallakurichi district.
- Hospitalizations: Around 82 others were receiving treatment in hospitals.
- Government Response: Chief Minister M.K. Stalin transferred the district Collector and suspended the superintendent of police following the incident.
- Police Deployment: The State deployed 2,000 police personnel around the district to control the situation.
- Historical Echo: This tragedy echoes a similar incident a year ago in the State’s Chengalpattu and Villupuram districts, where more than 20 people died after consuming spurious liquor.
Government Action
- Inquiry Commission: Mr. Stalin constituted a one-man commission headed by former High Court judge B. Gokuldas to inquire into the tragedy.
- State Control of Liquor Sales: Liquor sales in Tamil Nadu are controlled by the State through around 5,000 outlets.
- Immediate Response: The State’s immediate response included administrative changes and increased police presence.
- Preventive Measures: Measures were taken to prevent further incidents, including stricter enforcement of liquor laws.
- Public Safety Campaigns: The government initiated public safety campaigns to educate people about the dangers of consuming spurious liquor.
Production and Dangers of Spurious Liquor
Manufacturing Process
- Addition of Methanol: Spurious liquor is often homemade liquor to which methanol is added to strengthen the intoxicating effects and increase bulk volume.
- Methanol Sources: Methanol for spurious liquor is often purchased from factories and sold by arrack sellers.
- Distillation: Arrack is distilled from the fermented sap of the palm tree.
- Regulatory Limits: The Food Safety and Standards (Alcoholic Beverages) Regulations 2018 stipulate the maximum permissible quantity of methanol in different liquors.
- Economic Motives: The addition of methanol is economically motivated, as it is cheaper and increases the volume of liquor sold.
Deadly Effects
- Chemical Composition: Methanol (CH3OH) consists of one carbon atom bonded with three hydrogen atoms and one hydroxyl group.
- Toxic Metabolites: Methanol is metabolized to formaldehyde and then to formic acid, both of which are highly toxic.
- Health Consequences: Methanol poisoning can cause blindness, metabolic acidosis, cerebral edema, and death.
- Detection and Response: The presence of methanol in spurious liquor can be difficult to detect, and its effects are often delayed, complicating treatment.
- Prevalence: Methanol poisoning from spurious liquor is more common in poor societies in developing countries.
Why Methanol is Added to Liquor
Economic Incentives
- Cost-Effectiveness: Methanol is cheaper than ethanol, making it an attractive additive for illicit liquor producers.
- Increased Volume: Adding methanol increases the bulk volume of the liquor, allowing sellers to make more profit.
- Enhanced Intoxication: Methanol enhances the intoxicating effects of the liquor, giving it a stronger ‘kick’.
- Lack of Regulation: In areas with poor regulation and enforcement, methanol is added without fear of legal repercussions.
- Accessibility: Methanol is easily accessible from industrial sources, making it a convenient additive for illicit liquor production.
Metabolism in the Body
- Absorption: Methanol is completely absorbed via the gastrointestinal tract, with blood levels peaking within 90 minutes.
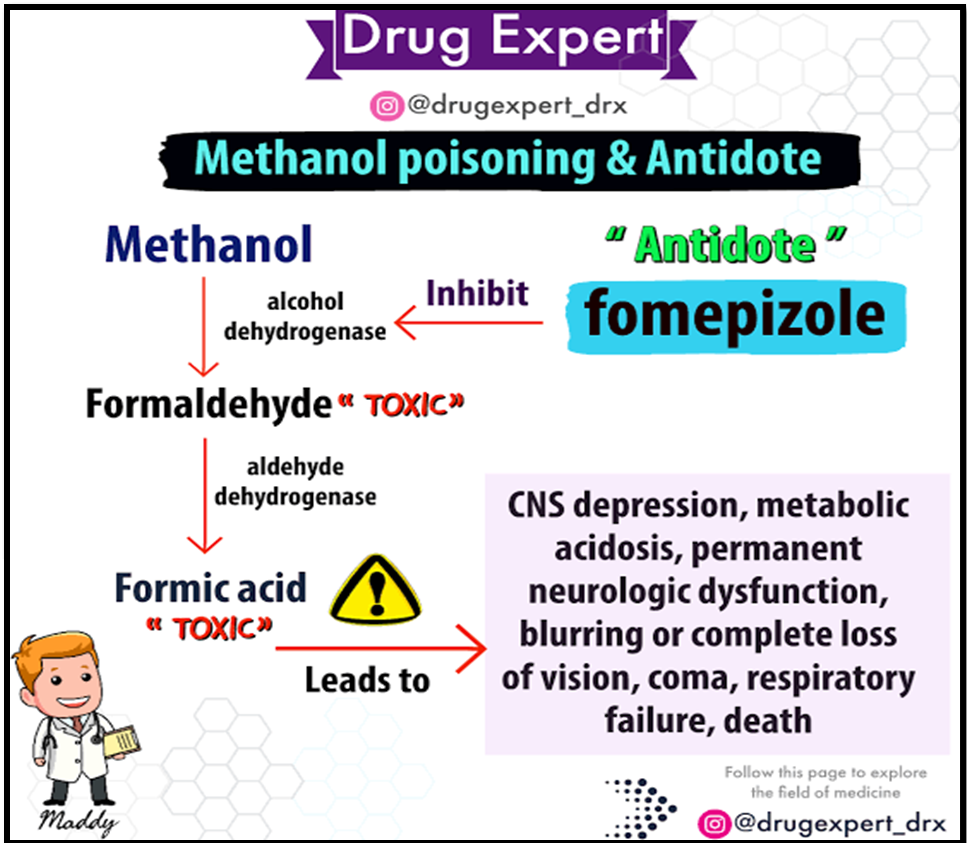
- Metabolic Pathway: Methanol is metabolized by alcohol dehydrogenase (ADH) enzymes in the liver to formaldehyde.
- Conversion to Formic Acid: Formaldehyde is further converted to formic acid by aldehyde dehydrogenase (ALDH) enzymes.
- Acid-Base Imbalance: The accumulation of formic acid leads to metabolic acidosis, disrupting the body’s acid-base balance.
- Long-Term Elimination: The body takes a long time to eliminate methanol completely, with significant amounts remaining even after 48 hours.
Effectiveness of Treatment
- Ethanol Administration: Pharmaceutical grade ethanol competes with methanol for ADH enzymes, preventing methanol from being metabolized to formaldehyde.
- Fomepizole: This antidote slows the action of ADH enzymes, allowing the body to excrete methanol before it is converted to toxic metabolites.
- Dialysis: Hemodialysis can be used to remove methanol and formic acid from the blood, reducing systemic toxicity.
- Folinic Acid: Administering folinic acid encourages the breakdown of formic acid into carbon dioxide and water.
- Expert Supervision: Both ethanol and fomepizole treatments require administration under expert medical supervision.
Limitations and Challenges
- Availability of Antidotes: Fomepizole is expensive, and pharmaceutical grade ethanol requires careful administration.
- Delayed Treatment: If methanol is ingested along with ethanol, the symptoms of poisoning may be delayed, complicating timely treatment.
- Healthcare Access: Access to appropriate healthcare and antidotes can be limited in poor or remote areas.
- Long-Term Damage: Even with treatment, long-term damage to the optic nerve, kidneys, heart, and brain may occur.
- Mortality Rates: Delayed or inadequate treatment increases mortality rates in methanol poisoning cases.
Conclusion:
By structuring the information under these subheads, the points offer a clear and organized explanation of methanol poisoning, the incident in Kallakurichi, the production and dangers of spurious liquor, the reasons for adding methanol to liquor, and the effectiveness of treatment.
Source:The Hindu
Mains Practice Question:
Discuss the dangers of methanol poisoning. Highlight the chemical process through which methanol causes toxicity in the human body. (250 words)
Associated Articles: