CONCERNS OVER COUGH SYRUP QUALITY IN INDIA:
Why in the News?
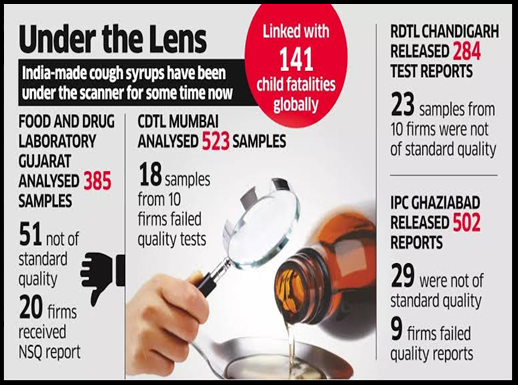
- Recent data from the Central Drug Standard Control Organisation( CDSCO )reveals that 6% of cough syrup samples from 54 Indian manufacturers failed mandatory quality tests for export until October this year.
- The CDSCO, emphasizes ongoing testing as a standard method to eliminate substandard products.
Source: The ET
India’s Global Pharmaceutical Leadership:
- India, a global leader in generic medicines, holds a 20% share in global supply by volume.
- India is known as the ‘pharmacy of the world,’ and is acknowledged for country’s significant role in supplying vaccines to 100 countries and medicines to 150 countries.
Export Screening Initiated:
- Screening of medicines destined for export began this year following concerns about the quality of cough syrups manufactured in India.
- Quality issues prompted the WHO to issue a medical product alert for a batch of Guaifenesin Syrup manufactured by an Indian company after reported incidents in Gambia, Uzbekistan, Cameroon.
Government Response and Industry Impact:
- The Central government responded by issuing a list of laboratories for pre-export testing to address contamination concerns.
- Contaminated syrups were reported to contain glycol and ethylene glycol, toxic substances potentially fatal, especially for children.
- India’s pharmaceutical sector, contributing 1.72% of GDP and valued at $50 billion, faces scrutiny to maintain its global reputation.
About Central Drug Standard Control Organisation (CDSCO):
- CDSCO is India’s central authority under the Drugs and Cosmetics Act of 1940.
- Operates within the Ministry of Health & Family Welfare, serving as the National Regulatory Authority (NRA) for India.
- Responsibilities include drug approvals, overseeing clinical trials, setting drug standards, and ensuring quality of imported drugs.
The government’s proactive testing measures seek to address these concerns and safeguard the nation’s pharmaceutical reputation.

 Source: The ET
Source: The ET

